The factor Xa (FXa) inhibitors apixaban, rivaroxaban and edoxaban, together with the thrombin inhibitor dabigatran, are collectively referred to as non-vitamin K antagonist (or direct) oral anticoagulants (NOACs). FXa inhibitors are widely used for stroke prevention in patients with nonvalvular atrial fibrillation,1 and for the treatment and prevention of deep vein thrombosis and pulmonary embolism.2,3 In patients with nonvalvular atrial fibrillation, NOACs have been reported to significantly reduce stroke or systemic embolic events compared to warfarin (relative risk [RR] 0.81, 95% confidence interval [CI] 0.73–0.91; p<0.0001), with reduction in haemorrhagic stroke (RR 0.49, 95% CI 0.38–0.64; p<0.0001) being the main driver of stroke reduction.4 In patients with venous thromboembolism, which includes deep vein thrombosis and pulmonary embolism, NOACs have comparable efficacy to vitamin K antagonists, with no significant difference in recurrence of venous thromboembolism (RR 0.88, 95% CI 0.74–1.05; p=0.46), fatal pulmonary embolism (RR 1.02, 95% CI 0.39–5.96; p=0.71) or all-cause mortality (RR 0.97, 95% CI 0.83–1.14; p=0.50).5
Bleeding is a feared complication of anticoagulant therapy. Warfarin-related bleeding is responsible for one-third of all hospitalisations for adverse drug events,6 and the mortality rate of patients hospitalised with vitamin K antagonist-related bleeding was 7.6%, with a 90-day mortality of 14.1%.7 Both in patients with nonvalvular atrial fibrillation and those with venous thromboembolism, NOACs are associated with a lower risk of major bleeding compared to warfarin. In particular, NOACs were associated with a lower risk of intracranial or life-threatening bleeding.4,8–11
Despite their demonstrated safety, the lack of a specific antidote may have limited the implementation of FXa inhibitors in clinical practice. However, andexanet alfa, the specific antidote for the reversal of FXa inhibitor activity, has recently been developed.12 The focus of this review will be on the position of andexanet alfa within the management of FXa inhibitor-associated bleeding.
Andexanet alfa as antidote for factor Xa inhibitors
Direct FXa inhibitors share a relative short elimination half-life of approximately 12 hours. Rivaroxaban and edoxaban are administered once-daily and apixaban is administered twice-daily. Renal clearance is 35%, 50% and 27%, respectively.13
Andexanet alfa is designed to reverse the direct and indirect anticoagulant activity of FXa inhibitors.14 Andexanet alfa is a modified, recombinant, inactive form of human FXa, with the ability to bind and sequester FXa-inhibitor molecules, thereby reducing its activity and restoring the amount of unbound endogenous FXa. However, andexanet alfa binds to tissue factor pathway inhibitors as well, which may lead to increased thrombin generation.
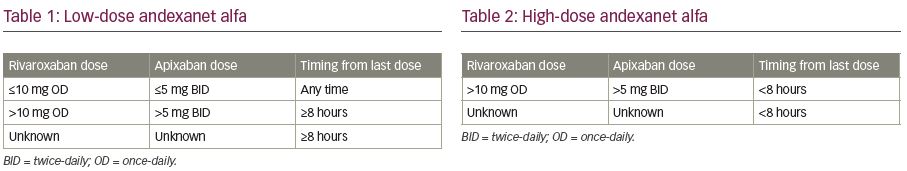
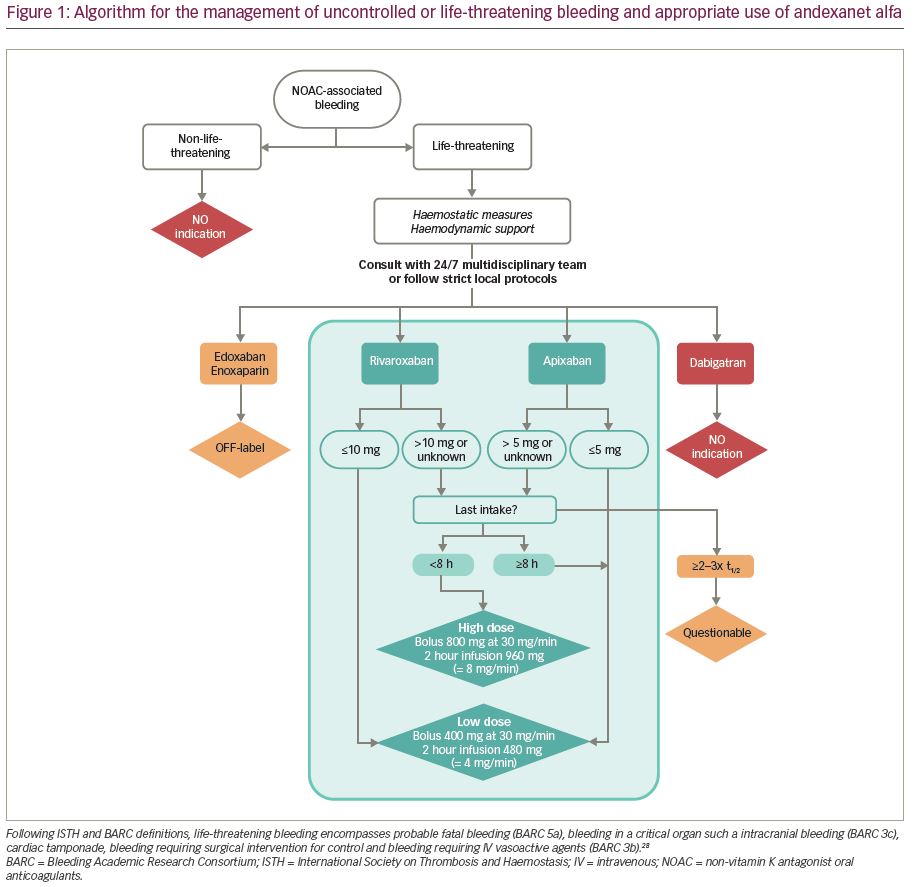
Andexanet alfa therapy is delivered by an intravenous (IV) bolus over a duration of 15–30 minutes, followed by an infusion of 2 hours. Prior to the initiation of andexanet alpha, the dose is adjusted according to the last dose of FXa inhibitor, and timing from the last intake of FXa inhibitor (Table 1 and Table 2; Figure 1).15 Low dose consists of an IV bolus of 400 mg at 30 mg/min, followed by an infusion of 480 mg at 4 mg/min. High dose consists of a double bolus of 800 mg followed by an infusion of 960 mg at 8 mg/min.
The dosing recommendations in the andexanet alfa product characteristics do not contain a maximum time from last dose of rivaroxaban or apixaban after which treatment with andexanet alfa could be considered futile. From a pharmacodynamic and clinical point of view, however, the clinical usefulness of treatment with andexanet alfa >2–3 times the half-lives after last dose of rivaroxaban (t½ 5–13 hours) or apixaban (t½ 13 hours), seems questionable.
In healthy volunteers, 50–75 years of age, and treated with apixaban or rivaroxaban, bolus administration of andexanet alfa reduced anti-FXa activity and restored thrombin generation within 2–5 minutes.16 When andexanet alfa was administered as bolus plus infusion, consistent with its half-life of ~1 hour, the effects were sustained and then waned after 2 hours. In a subgroup of participants, transient increases in levels of D-dimer and prothrombin fragments 1 and 2 resolved within 24–72 hours without thrombotic events.16
ANNEXA-4 was a single-group cohort study that evaluated the clinical utility of andexanet alfa in patients who experienced acute major bleeding within 18 hours after administration of apixaban (54%), rivaroxaban (40%) or enoxaparin (6%).17 Sixty-four percent of patients suffered from intracranial bleeding and 26% from gastrointestinal bleeding. Patients were treated with a bolus of andexanet alfa, followed by a 2-hour infusion. Treatment with andexanet alfa resulted in a 92% reduction of anti-FXa activity. In 85% of patients with gastrointestinal bleeding and in 80% of patients with intracranial bleeding, ‘excellent’ or ‘good’ haemostatic efficacy was observed at 12 hours, as adjudicated according to prespecified criteria. Within 30 days, 14% of the patients had died. Thrombotic events occurred in 10% of patients, none of whom had yet restarted oral anticoagulation.17
Based on the results of the ANNEXA-4 study, the use of andexanet alfa has been conditionally approved by both the US Food and Drug Administration and the European Medicines Agency for adult patients treated with a direct FXa inhibitor (apixaban or rivaroxaban) when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.15,18 However, the risk–benefit ratio of andexanet alfa in comparison to usual care remains to be determined. As this is a condition for accelerated approval in the United States, a randomised clinical trial is currently ongoing to determine the efficacy and safety of andexanet alfa compared to usual care in patients presenting with acute intracranial haemorrhage within 12 hours of symptom onset and within 15 hours of taking an oral FXa inhibitor, with an estimated completion date in 2023.19
Use of andexanet alfa in the setting of mild or non-life-threatening bleeding, for patients undergoing urgent or immediate high-risk surgery, or use of andexanet alfa for other FXa inhibitors than apixaban or rivaroxaban, is off-label and is the subject of ongoing studies. In preclinical studies, andexanet alfa effectively reversed the anticoagulant effect of other FXa inhibitors.20,21 Clinical data of andexanet alfa in patients requiring urgent surgery and receiving apixaban, rivaroxaban, edoxaban or enoxaparin within 15 hours prior to the start of surgery, are currently being evaluated in a prospective open-label study.22
Practical considerations
With approval of idarucizumab in 2015,23 and andexanet alfa in 2019, effective direct anticoagulant reversal agents are now available for patients taking dabigatran, or apixaban or rivaroxaban. With the widespread availability of these effective anticoagulant reversal agents, the correct use of these agents in real-world care needs special attention. The decision to use reversal agents for NOACs is most likely to be made in the acute critical care setting, in which prompt decisions are required. Inappropriate use unnecessarily exposes patients to (thrombotic) risks, and increases healthcare costs.
The clinical settings in which idarucizumab is used in real-life care, in all probability, are a good reflection of the clinical situations in which andexanet alfa will be used as well. The appropriate usage of idarucizumab in daily clinical practice was evaluated in a retrospective observational study by Van der Wall et al.24 Between 2016 and 2018, 88 patients were treated with idarucizumab in 12 Dutch hospitals. The number of administrations of idarucizumab varied from 1–14 during the 2-year study period. Inappropriate use of idarucizumab occurred in 25 of the patients (28%): in 14 patients for an intervention that could have been delayed for at least 8 hours, and in 11 patients for bleeding complications that were not considered uncontrollable. Three patients (5.7%) had
non-detectable dabigatran plasma levels, in two of these patients the last drug intake was >72 hours previously and one patient used rivaroxaban.
These data demonstrate the importance of introducing a local treatment protocol for the appropriate use of direct anticoagulant reversal agents, and correctly determining the severity of the bleeding event or the urgency and bleeding risk of surgical procedures, and the importance of assessing the type, dosage and time of the last intake of the NOAC.
Routine coagulation tests (prothrombin time, activated partial thromboplastin time and international normalised ratio) do not provide an accurate assessment of the anticoagulant effects of FXa inhibitors. However, in case of serious bleeding, coagulation tests may help the clinician to support haemostasis. Anticoagulant effects can be measured via specific coagulation assays developed for the quantification of NOAC plasma levels. Most routine coagulometers are capable of measuring NOAC plasma levels within ≤30 minutes.25 Healthcare institutions are recommended to consider 24/7 availability of these tests for emergency situations. Absence of anti-Xa activity with chromogenic assays excludes clinically relevant drug levels. Protamine sulphate and vitamin K are not expected to affect the anticoagulant activity of rivaroxaban or apixaban. There is limited experience with tranexamic acid or prothrombin complex concentrates (PCCs).
In a meta-analysis of 10 single-arm studies in 340 patients, and a retrospective observational study of 31 patients who received PCC for FXa inhibitor-related major bleeding, effective haemostasis was reported in 69% and 81%, respectively.26,27 Based on non-placebo-controlled reports, however, it is difficult to determine whether PCC, in addition to cessation of direct oral FXa inhibitor, is more effective than cessation of direct oral FXa inhibitor alone. To the best of our knowledge, there are no randomised trials comparing PCC to andexanet alfa. The decision to administer andexanet alfa should be made in consultation with a multidisciplinary team who should be available 24/7 and consist of a coagulation expert, a cardiologist specialising in anticoagulation, a vascular internal medicine physician and a clinical chemist.
Local protocols should be available describing the treatment of patients using FXa inhibitors with uncontrolled or life-threatening bleeding, including haemostatic measures, haemodynamic support and the use of andexanet alfa. According the Committee for Medicinal Products for Human Use, the dose of andexanet alfa is first determined by the last dose of rivaroxaban or apixaban before initiation of andexanet alfa. By default, patients treated with apixaban for stroke prevention in atrial fibrillation will always use an apixaban dose ≤5 mg. Hence, these patients will always receive the low dose of andexanet alfa. Likewise, patients using rivaroxaban ≤10 mg will always receive the low dose andexanet alfa. In patients using higher doses of apixaban or rivaroxaban, the timing from last intake before initiation of andexanet alfa determines the dose of andexanet alfa (Figure 1).28