Introduction: Sudden death due to ventricular arrhythmias is a major cause of mortality in adults with repaired Tetralogy of Fallot (ToF). Consensus guidelines recommend implantable cardioverter defibrillators (ICD) in selected ToF patients with multiple risk factors for ventricular arrhythmia or following sustained ventricular tachycardia (VT) or cardiac arrest. However, data regarding ICD outcomes in this population are limited.
Methods: Retrospective observational study of adults with repaired ToF who received ICD at a single centre between 2004–2018. Objectives were 1) to determine the incidence and clinical predictors of appropriate ICD shocks; and 2) to assess the frequency of complications and inappropriate ICD shocks.
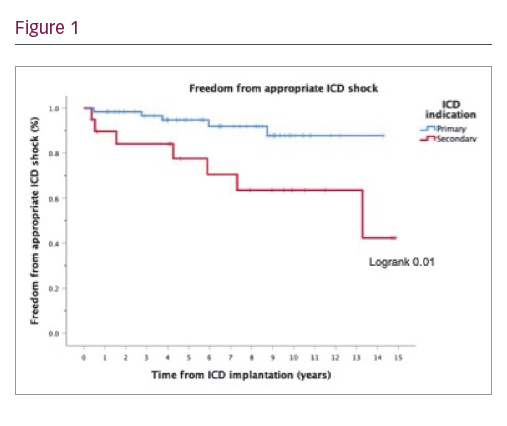
Results: Eighty-four patients received ICD (73.8% male; mean age 44.8 ± 14.0 years at implant; 33.7 ± 11.6 years after ToF repair). ICDs were for primary prevention in 64 patients (76.2%) and for secondary prevention in 20 patients (23.8%). Indications for secondary prevention ICD were sustained or haemodynamically compromising VT for 14 patients (70%) and ventricular fibrillation for 6 patients (30%). Indications for primary prevention ICD were multifactorial and included QRS ≥180 ms in 27 (32.1%), non-sustained ventricular tachycardia VT in 23 (27.4%), inducible sustained VT in 23 (27.4%), RV ejection fraction ≤35% in 18 (21.4%), LV ejection fraction ≤35% in 7 (8.3%) and syncope in 10 patients (11.9%). ICD devices were single-chamber in 3 (3.6%), dual-chamber in 51 (60.7%), subcutaneous in 2 (2.4%) and cardiac resynchronisation therapy ICD in 28 patients (33.3%). Twelve patients (14.3%) received appropriate ICD shocks during a median follow-up of 7.8 years (593 patient years). 85.7% of appropriate ICD shocks were for monomorphic VT with mean detected cycle length of 279 msec. Appropriate shocks were more frequent in patients with secondary prevention ICD (35% of secondary versus 7.8% of primary prevention group, p=0.024). No clinical predictors of appropriate ICD shocks were identified in the primary prevention group. Inappropriate ICD shocks occurred in 17 patients (20.2%), predominantly due to atrial tachycardia. Annual event rates of appropriate and inappropriate ICD shocks were 3.7% and 5.6% respectively, which is comparable to results of previous studies. Device-related complications occurred in 16 patients (19.0%): 3 acute procedural complications, 8 late lead failures, 3 device-related infections, 2 device erosions and 1 thrombus. Fifteen patients (17.9%) required device re-intervention and 38 (45.2%) generator box change. 15 patients (17.9%) died and 1 patient was transplanted during follow-up.
Conclusions: Adults with ToF and ICD receive a modest rate of appropriate ICD shocks (3.7% per annum). However, there remains a considerable burden of device-related complications and inappropriate shocks.