Background: There are over 740 bradycardia pacemaker patients per million in Western Europe. Pacemakers are a safe and effective treatment to bradycardia to increase longevity and improve quality of life. Cross-sectional and retrospective studies have suggested left ventricular systolic dysfunction (LVSD) and heart failure (HF) are common in the pacemaker population. Personalised pacemaker programming in an observational cohort has successfully reduced pacing requirements leading to improved left ventricular (LV) function without affecting quality of life. No randomised trial has yet explored these effects.
Methods: This was a randomised double-blind parallel study of personalised reprogramming versus standard care on ventricular pacing burden, LV function, and pacemaker longevity. The study was approved by ethical committee and registered on clinicaltrials.gov. Patients were eligible if they had a bradycardia pacemaker for indications other than third degree heart block and adequate echocardiographic imaging. All participants gave written consent, received a transthoracic echocardiogram, pacemaker interrogation, blood draw, clinical examination and had a clinical history taken alongside quality of life assessment. Participants were contacted to ensure acceptability and safety of reprogramming at 1 week by a blinded healthcare professional, and followed up at 6 months.
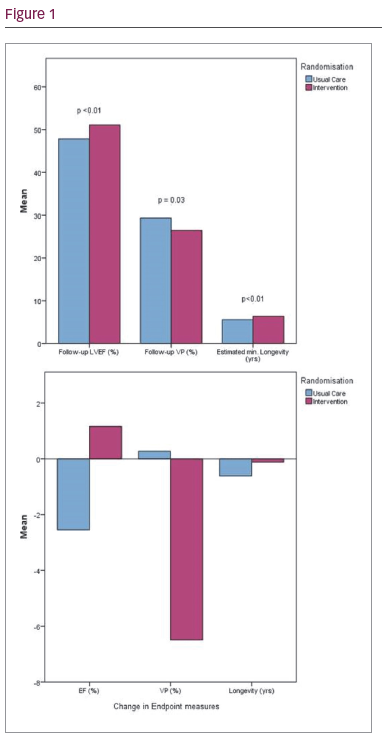
Results: One hundred patients were recruited and assigned 1:1 to reprogramming intervention or standard care. Study groups were well matched for age, ventricular pacing burden, and LV function (as measured by ejection fraction). Personalised reprogramming involved assessment and manipulation of the base rate (44%), sleep, rest or hysteresis rates (68%), mode (8%), rate-response (38%), atrioventricular search (4%), autocapture (36%), lead outputs (74%) and electrogram storage (10%) as appropriate and based upon a previously published reprogramming protocol. Eight patients withdrew due to illness or the onset of a life-threatening co-morbidity, 1 patient was upgraded to a biventricular device. Seventy-one participants were male with a mean age of 75.8 years, right ventricular (RV) pacing burden of 36.2% and ejection fraction of 49.7%. Intervention compared to usual care significantly reduced RV pacing burden (–6.5 ± 1.8% versus
–0.21 ± 1.7%; p<0.01), improved ejection fraction (+2.1 ± 6.9% versus –0.70 ± 5.6%; p=0.03), and preserved minimum battery longevity by approximately 6 months (–1.4 ± 7.8 months versus
–7.3 ± 12.5 months; p<0.01). 1 patient returned with worsening symptoms but was subsequently upgraded due to deteriorating heart failure. Quality of life analyses are underway.
Conclusion: Personalised pacemaker reprogramming, even in the era of RV pacing avoidance algorithms, can reduce unnecessary RV pacing, improve left ventricular ejection fraction and significantly preserve battery life in pacemaker patients.