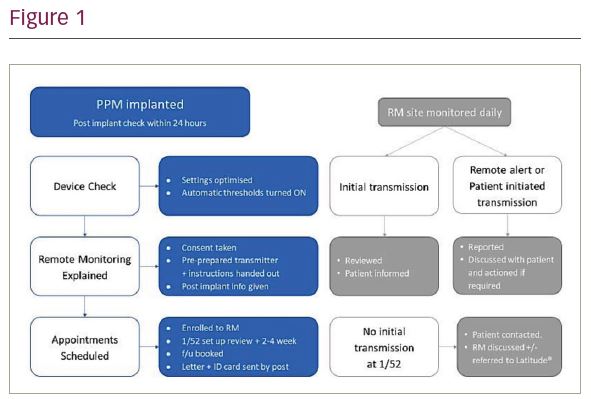
Background: The 2015 Heart Rhythm Society consensus statement on remote interrogation and monitoring of cardiovascular implantable electronic devices (CIEDs) recommends that all CIEDs be checked through direct patient contact 2-12 weeks post implant. The COVID19 pandemic forced our pacemaker (PPM) service to deviate from this recommendation. We enrolled patients to remote monitoring (RM) from implant and performed this check ‘remotely’ using RM technology.
We audited this change in practice to determine if we should continue with our approach post COVID and to look for areas of service improvement.
Method: Patients requiring a PPM between 23rd March and 4th June were implanted with a Boston Scientific PPM and enrolled to the Latitude® remote patient management system. The protocol followed is displayed in Figure 1. We retrospectively reviewed PPM reports and RM transmissions to assess:
- time to transmitter set up
- patient compliance
- alerts received
- alerts requiring intervention
- effect of programming automatic thresholds on from the day
of implant - device function at the 2-12 week check
For comparison a control group of patients was collected where settings optimised at in-person 2-12 week checks were reviewed.
Results:
- 83 PPM implants were performed. 57% (n=47) were dual chamber pacemakers (DDD), 24% (n=20) were single chamber pacemakers and 19% (n=16) were generator changes.
- 81% (n=67) were implanted for syncope or pre-syncope.
- 57% (n=47) for AV block, 20% (n=17) for sinus node disease and 14% (n=12) for permanent AF with bradycardia.
- Median patient age at implant was 78 years old, range 30 to 104
years old. - 93% (n=77) patients were consented to RM and received a RM transmitter on the day of implant.
- 64.9% (n=53) of these patients had set up RM in ≤ 3 days of implant.
- 97.5% (n=81) of all patients had set up RM within 2 weeks of implant.
- 23 remote alerts were received.
- 3 alerts in 2 patients resulted in early detection of lead complications requiring intervention.
- 52% (n=12) of alerts could have been avoided by better tailoring of patient alerts at implant.
- 84% (n=70) patients had automatic thresholds turned on in all system leads on the day of implant. 100% (n=70) of these patients had normal pacemaker function and appropriately set outputs at the 2-12 week follow-up.
- In a control group of 99 patients implanted with a PPM between September and December 2019 who attended for a 2-12 week check 60% (n=59) had their lead outputs optimised at this check.
Conclusion: Enrolling patients to RM at implant was possible with high patient compliance and was beneficial to patients in detecting early lead complications requiring intervention. Tailoring remote alerts at implant may help reduce the number of remote alerts received by >50%. Automatic threshold tests are reliable and safe to program on from implant when combined with RM and may reduce the need for patients to attend a 2-12 week post implant check.