Introduction: Left ventricular outflow tract obstruction (LVOTO) is reported in up to two-thirds of hypertrophic cardiomyopathy (HCM) patients. This can cause symptoms of dyspnoea, chest pain, pre-syncope, and syncope. Disopyramide is a negative inotrope that can reduce the pressure gradient created by LVOTO. Disopyramide has a class 1B recommendation for LVOTO in the European Society of Cardiology HCM guidelines and can be trialled in symptomatic patients before proceeding to more invasive treatment.
Purpose: To evaluate the safety and tolerability of disopyramide initiation in an inpatient and outpatient setting.
Methods: A total of 42 patients with obstructive HCM were started on disopyramide in our centre from 2017 to 2022. From 2017 to 2020, all disopyramide initiation required 2 days’ admission with telemetry monitoring, electrocardiogram three times a day, and inpatient echocardiogram. Patients were started on 100 mg three times a day and uptitrated to 250 mg twice a day by Day 2 if there were no rhythm abnormalities or significant conduction or repolarisation changes on electrocardiogram (ECG). We started outpatient initiation from 2020 with a starting dose of 100 mg three times a day and titrating to 250 mg MR twice a day within 3 weeks. All patients had an ECG on the day of initiation, 1 week post initiation and 2 weeks post initiation.
Results: In our inpatient cohort (n=31), there was no significant change in heart rate (HR 73 ± 1 4 bpm to 71 ± 13 bpm). There was some non-significant delay in conduction when peak disopyramide dose was achieved: PR (168 ± 19 ms to 184 ± 27 ms), QRS (104 ± 21 ms to 113 ± 26 ms). Repolarisation measures were also delayed in a non-significant manner: QT (408 ± 38 ms to 439 ± 39 ms) and corrected QT (445 ± 37 ms to 475 ± 41 ms). No patient had QTc >500 ms.
Our outpatient cohort (n=11) had similar findings with HR (67 ± 17 bpm to 66 ± 15 bpm), PR (175 ± 34 ms to 193 ± 35 ms), QRS (113 ± 25 ms to 117 ± 22 ms), QT (428 ± 57 ms to 453 ± 50 ms), and corrected QT (442 ± 25 ms to 467 ± 22 ms).
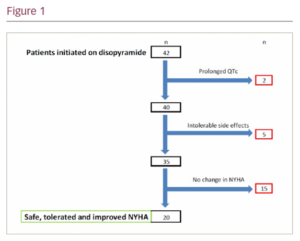
A total of 11 patients had side effects following initiation and seven patients (17%) had to discontinue treatment. The most common side effects reported were dry mouth (n=6), constipation (n=7), urinary symptoms (n=3), visual changes (n=4) and pre-syncope (n=1). Prolonged QTc >500msec was seen later in two patients on follow-up.
There was no sudden cardiac death or syncope in our patient cohort. For patients who continued on disopyramide (n=35), there was a decrease in the mean New York Heart Association (NYHA) functional class from 2.06 ± 0.7 to1.35 ± 0.5 on a mean follow-up of 171 days. A total of 20 patients (57%) who continued on disopyramide improved by at least one NYHA class (Figure).
Conclusion: Our data suggest disopyramide is effective in improving symptoms of dyspnoea and is safe in patients with obstructive HCM. 17% of patients stopped disopyramide due to side effects and 5% stop due to QTc >500 ms. ❑