Introduction: In February 2020 the British Heart Rhythm Society (BHRS) published updated standards for the follow up of cardiac rhythm devices which included new standards for report writing following device interrogations. Creation and adherence to these guidelines can provide structured device reports to facilitate the safe delivery of high-quality patient care. These guidelines were used to create a departmental standard operating procedure (SOP) at our centre and disseminated amongst Cardiac Physiologists/Scientists. This study assesses compliance of device follow up reports to the departmental SOP.
Methods: All pacing follow-up reports over a five-month period were extracted from our Institutional implantable device database at the Royal Brompton Hospital (n=1791). A random number generator was used to select 200 follow up reports to include in the analysis. Follow up reports and programmer/transmission data were systematically reviewed to assess compliance with departmental SOP criteria including: follow up check type, documentation of patient symptoms, wound status, presenting rhythm, underlying rhythm, battery and lead status, pacing percentages, heart rate histograms, arrhythmia episodes/burden, programming changes and the individual who completed the report. Documentation of patient symptoms and underlying rhythms were excluded for remote monitoring reports.
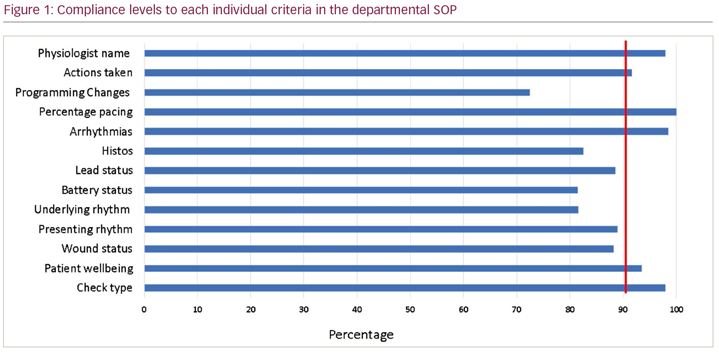
Results: Of the 200 reports analysed, 102 were via remote monitoring (51%) and 98 were in person clinic follow up checks (49%). All follow ups were performed by Cardiac Physiologists or Cardiac Scientists. There was an overall compliance of 89.5% with local SOP reporting standards. Poor compliance was noted for documentation of the presenting rhythm (89%), underlying rhythm (81%) and heart rate histograms (82.5%). Compliance was higher for patient symptoms (93.5%), pacing percentages (100%), arrhythmias (98.5%) and the individual performing the check (98%). These criteria are all demonstrated in Figure 1. Of 17 reports for the first post implant check, 15 documented a wound assessment had taken place (88.3%). Forty-four reports (22%) documented programming changes or a requirement for further action (e.g. medication review/referral for other tests). Nine reports on remote transmissions required an action to be taken by the physiologist or clinician (8.82%).
In our single centre experience, there is a good compliance rate of follow up reports with departmental and national standards. Particular attention should be paid to accurate documentation of the presenting and underlying rhythm as well as heart rate histograms. An additional audit, with more post implant checks evaluating compliance with documentation of wound assessments would also be useful. Audits of device reporting compliance with SOPs should be completed regularly to ensure high compliance levels and the standardisation of reporting.