The atrial transseptal puncture (TSP) technique is an indispensable procedure in the invasive cardiac catheterisation laboratory. TSP was initially developed in 1959 by Ross, Braunwald and Morrow at the National Institutes of Health, to allow for catheter access to the left atrium (LA) and left ventricle (LV) for the evaluation of valvular heart disease.1 However, today the need for procedural access to the left heart has expanded to include variety of cardiac conditions, which include: electrophysiological mapping of the left heart, catheter ablation of tachycardias, percutaneous mitral valvuloplasty, left atrial appendage exclusion and haemodynamic measurements in chambers with entry limited by prostheses.2 Thus, in recent years there has been an exponential increase in the use of TSP for the treatment of left-sided cardiac disease.
Currently, the most common technique used for TSP is the Brockenbrough technique, which utilises fluoroscopy for sheath placement and septal puncture.3 However, with new imaging technologies including electroanatomical mapping (EAM) and intracardiac echocardiography (ICE), the risk-to-benefit ratio of using fluoroscopic TSP versus standard procedural risk remains unknown. There have been several recent reports of zero-fluoroscopy approaches to the ablation of atrial arrhythmias.4–13 However, the combined use of ICE and EAM to map the interatrial septum and set the TSP needle, has not been described in these procedures. Here, we describe a feasibility study to evaluate a completely zero-fluoroscopy technique for TSP (ZFTSP), in patients undergoing ablation for atrial fibrillation (AF), that takes advantage of the ThermoCool® SmartTouch™ catheter (Biosense Webster Inc., California, US) ablation catheter as a non-traumatic guide for imaging the fossa ovalis, and setting the transseptal sheath for puncture. This technique advances the concept of combined use of ICE and EAM for TSP, and contributes towards a zerofluoroscopy approach to interventional electrophysiology procedures. Finally, we place this technique in perspective of larger, randomised trials examining this important radiation-saving procedure.
Methods
Study population and design
We performed a retrospective analysis of patients with paroxysmal or persistent AF who previously elected to have a pulmonary vein isolation procedure due to failure of at least one antiarrhythmic drug (AAD). Outcomes of patients who received fluoroscopy as part of the TSP were compared with patients in which fluoroscopy was not used due to the use of the ZFTSP technique. Seven ZFTSP patients were compared with eight age-matched TSP controls with fluoroscopy. The study protocol was reviewed and approved by the Institutional Review Boards of the Texas Heart Institute at Baylor College of Medicine.
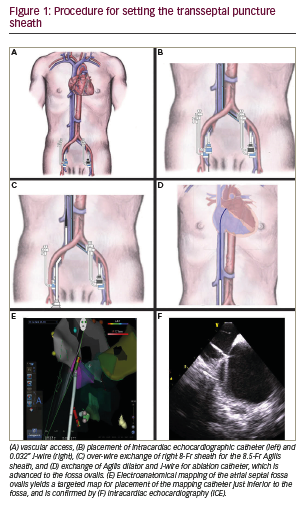
Procedure technique The technique for ZFTSP requires a ThermoCool SmartTouch catheter, Carto® mapping system (Biosense Webster Inc., California, US), Agilis™ Steerable Introducer (St. Jude Medical, Saint Paul, Minnesota, US) or SL-1 transseptal sheath (St. Jude Medical, Saint Paul, Minnesota, US), transseptal needle appropriately sized for the respective sheath, an 0.032” guidewire, ICE catheter (Biosense Webster Inc., California, US) with Vivid™ Echocardiography System (GE Healthcare, Chicago, US) and a decapolar coronary sinus (CS) catheter. First, 8-Fr short and 9-Fr long left common femoral venous (CFV) sheaths are placed without fluoroscopy, as well as two 8-Fr short sheaths in the right common femoral vein (Figure 1A). A decapolar catheter is placed from the left 8-Fr sheath to the CS. Next, an AcuNavTM Intracardiac Echo Catheter (Biosense Webster Inc., California, US) is placed from the left 9-Fr long sheath to the right atrium. The echocardiographic window was then focused on the fossa ovalis, which was located on the inter-atrial septum, posterior to the aorta and anterior to the left pulmonary veins. The placement of the decapolar CS catheter and intracardiac echo catheter can be placed entirely using Carto mapping, and fluoroscopy is not necessary for this portion of the procedure. Often, the CS can be clearly seen by the intracardiac echo catheter, and this may serve as a basis to place the CS catheter.
Through one of the right CFV sheaths, a 0.032” guidewire is passed up the inferior vena cava (IVC) approximately 40 cm, and the sheath is exchanged over wire to a transseptal sheath (either Agilis or SL-1, Figure 1B). The transseptal sheath is advanced into the right CFV, to the IVC about 20–40 cm, and then wire and dilator are removed and the sheath is flushed with heparinated saline (Figure 1C). A flushed ThermoCool SmartTouch catheter is inserted via the introducer tool into the transseptal sheath, and is advanced by 3D Carto imaging to the heart (Figure 1D). The catheter becomes visualised when it exits the sheath and becomes a guide to insertion of both the catheter and sheath into the right atrium (Figure 1E–F). By intracardiac echo guidance and Carto sound mapping, the inter-atrial septum and fossa are visualised, and anatomy is recorded. Additionally, finer detail of the septum is mapped with the ablation catheter.
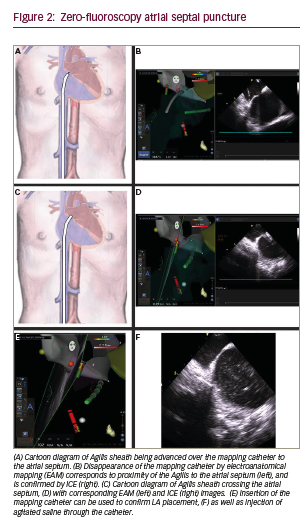
Once the septum is adequately defined by ICE and mapping such that the fossa ovalis and surrounding myocardium is distinct, the ICE catheter and ablation catheter are fixed, and then the transseptal sheath is advanced over the ablation catheter to the inter-atrial septum (Figure 2A–B). There are two ways to confirm adequate placement of the sheath on the lower third of the septum. First, by intracardiac echo, the sheath sideport is connected to heparinated saline with a flow regulator. Flushing the flow regulator produces microbubbles of saline that can be visualised on echo. Second, with the ablation catheter fixed on the desired area of the septum, advancing the sheath will result in eventual visual loss of the catheter tip on Carto mapping, which coincides with “housing” of the ablation catheter in the sheath and placement of the sheath on the septum. Next, with the sheath position fixed, the ablation catheter is removed and the sheath dilator and transseptal needle are advanced to the tip under intracardiac echo guidance (Figure 2C).
Gentle forward manual pressure is applied to sheath, dilator and needle until the needle crosses the septum by echo and microbubbles are seen on echo (Figure 2D–F). Often, very little pressure is required to puncture the septum given the more exact placement of the sheath by dual imaging, than by fluoroscopy alone. Additionally, left atrial pressure is recorded to confirm left atrial placement and to prevent pressure dampening suggestive of potential perforation risk. Pressure is monitored while the needle is fixed and the dilator and sheath are advanced approximately 2 cm. The dilator is then fixed and the sheath is advanced another 2–3 cm under echo guidance until the sheath is visualised to cross the septum.
Statistical analysis
A two-tailed Student t-test was used to compare continuous variables including age, body mass index (BMI), AF duration, procedure time, and fluoroscopy time between the ZFTSP and standard TSP groups. Fisher’s exact test was used to compare occurrences of complications and recurrence rate within one year. A P value of ≤0.05 was considered statistically significant.
Results
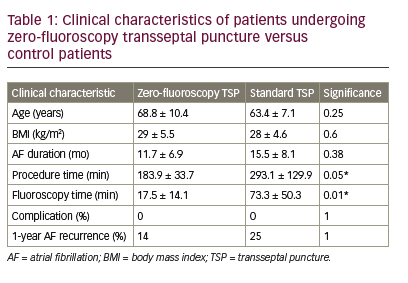
A total of seven patients received ZFTSP. The success rate of performing ZFTSP was 100% (7/7), and there were no redo ZFTSP procedures required. There were no significant baseline differences in age, BMI, or pre-procedural AF duration versus eight age-matched controls. ZFTSP procedure time was 183.9 ± 33.7 minutes versus 293.1 ± 129.9 in controls (Table 1, p=0.05). Fluoroscopy time in ZFTP patients was 17.5 ± 14.1 minutes versus 73.4 ± 50.3 in controls (p=0.01); of note, the control group included one patient with prior Tetralogy of Fallot repair, and one patient with prior mechanical mitral valve repair. There were no immediate complications among the cohort; specifically, there was no evidence of the following: post-procedural bleeding, pericardial effusion, tamponade, oesophageal damage, atrio-oesophageal fistulas, rehospitalisations and death. One-year post-procedural follow-up showed one of seven ZFTSP patients (14%) developed AF recurrence, which occurred 1 month postprocedure, while the remaining six patients (86%) were AF-free at 1 year. By comparison, two of eight TSP control patients (25%) developed AF recurrence within the year following the procedure.
Discussion
The main finding of our feasibility study is that a combination of ICE and intracardiac Carto mapping may be used to accurately “set” the transseptal sheath, which may then cross the inter-atrial septum using
the usual dilator and needle, without the use of fluoroscopy. This is made possible by technological improvements in EAM, force-sensing ablation catheters, and ICE. The use of this technique bypasses one of the major limitations of fluoroscopy, namely its inability to accurately identify endocardial structures in detail,14 and it also reduces procedural exposure to radiation.
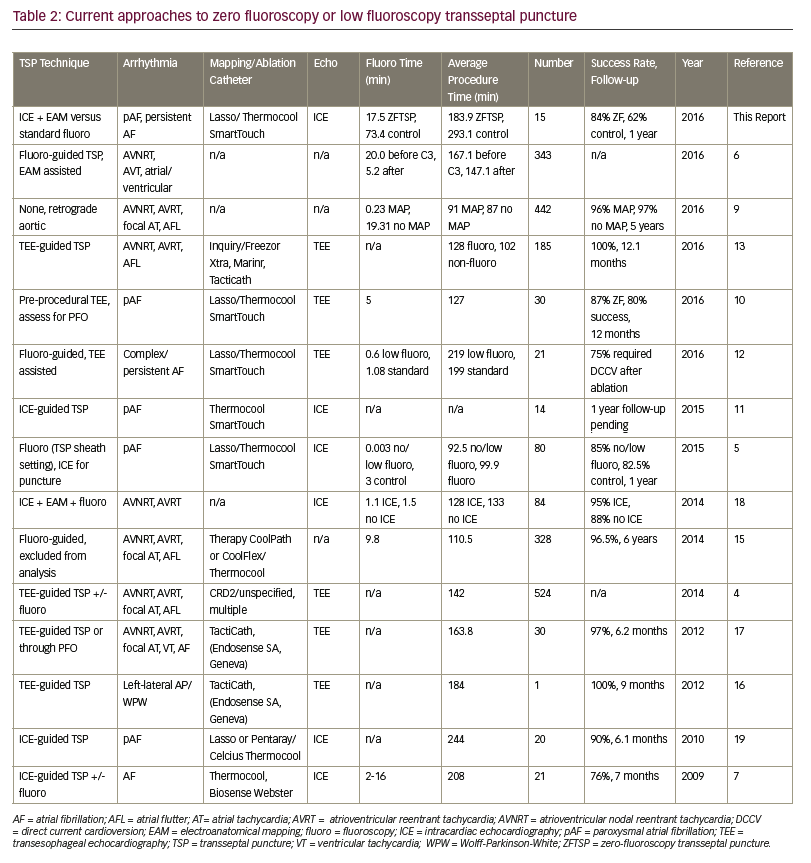
Previous zero-fluoroscopy approaches to the transseptal technique have used a variety of approaches to TSP (Table 2).4–7,9–13,15–19 Kühne et al. advocate using transesophageal echocardiography (TEE) prior to TSP access,10 whereas several groups describe intra-procedural TEE-guided TSP access with a high degree (97–100%) of AF-free event rates during respective follow-up periods.13,16,17 Other groups have described TEE-guided TSP in combination with fluoroscopy to minimise radiation exposure, however reported outcomes from this approach demonstrated comparatively reduced AF-free success rates (75–85%).4,5,12 No groups have reported EAM-only ZFTSP access, but Clark et al. have demonstrated that EAM-guided TSP access combined with fluoroscopy is feasible.6 Reddy et al. demonstrated that ICE-guided ZFTSP yielded 90% AF-free success rates at 6.1 months; Montgomery et al. follow-up results using the same approach are pending at this time.11,19
The benefits of our ZFTSP approach to existing techniques include EAM of the relevant portions of the inter-atrial septum, improved accuracy of transseptal sheath placement and anatomical mapping of the site of puncture, which may be useful for re-establishing transseptal access if the sheath is withdrawn from the LA. Additionally, our ZFTSP technique contributes to the long-term goals of reducing ionising radiation exposure to both patient and staff to “As Low As Reasonably Achievable” (ALARA).20 The risks of chronic radiation exposure have been well established in both patients and medical professionals.21–24 The risk to interventional cardiologists is most likely the highest among medical professionals because they have been noted to receive more chronic total radiation than any other medical staff using x-ray imaging.25,26 Cancer risk among interventional physicians using fluoroscopy remains a controversial topic, but it is receiving increasing attention within the medical literature. For example, recent studies have shown that medical professionals utilising fluoroscopy have an increase in the number of glioblastoma mulitforme, meningiomas and astrocytomas to be associated with their radiation exposures.27–30 Additionally, a recent cohort has reported 31 cases of interventionists with brain cancer.31 These observational studies do not yet prove that interventional procedures cause head and neck cancers among medical professionals, but do highlight a growing concern that will need to be addressed in prospective, randomised trials.
Zero fluoroscopy transseptal techniques may help to alleviate the orthopaedic strain of wearing leaded shield protection. In 2004 Society of Cardiac Angiography and Interventions Survey, nearly half of the respondents reported spinal disease and more than one-third indicated their spine problems caused them to miss work.32 The prevalence of spinal disc disease among interventional cardiologists was well described by Ross et al. who showed that cardiologists, versus orthopaedic surgeons and rheumatologists, had the greatest number of complaints for axial skeletal injuries and number of work-days missed secondary to back and neck pain.33 Efforts to reduce orthopaedic strain and improve radiation protection also include the radiation protection cabin34 and the Biotronik Zero-Gravity System (Biotronik, Berlin, Germany). By implementing a fluoroscopy-independent method for cardiac procedures, and utilising an external radiation framework when fluoroscopy is absolutely necessary, interventional cardiologists may gain significant advantage by relieving the risk of orthopaedic complications.
Limitations of our study include the risk of the “set” sheath migrating up the septal wall after the mapping catheter is removed in exchange for the transseptal dilator and needle. Although this did not occur during our performance of this technique, this is a legitimate concern that may increase risk of perforation if not addressed. This risk may be mitigated by evaluating the position of the sheath on ICE; should there be a change in sheath position detectable on ICE, the sheath may be reset by reintroducing the mapping catheter. Second, in this small group of patients, there were no significant complications, though the technique should be validated and refined in larger cohorts in the future. Additionally, the TSP fluoroscopy times in this small group of patients were higher than previously reported values;5,9,10,12 this may be due to the assistance of general cardiology fellows, who generally require longer fluoroscopy times for TSP than an experienced cardiologist, and the addition of patients with either paroxysmal or persistent AF, the latter of which may require longer procedure times and fluoroscopy use.
Conclusion
There are several advantages to the zero-fluoroscopy transseptal technique over the current standard of practice. One of the main advantages is the elimination of radiation exposure to both patient and operator. Secondly, the use of the SmartTouch pressuresensing catheter allows the operator to monitor distal catheter tip pressure accurately and in real-time to avoid inadvertent perforation. Lastly, the improved EAM technique coupled with ICE allows the operator to better visualise structures, including endocardial structures, which is only partially achievable with fluoroscopy. Our technique takes advantage of three sources of non-fluoroscopic information (echocardiographic anatomical data, 3D anatomical septal mapping and distal catheter tip force sensing) to accurately determine the best site for puncture, reliably deliver the transseptal sheath to this site, and accurate placement of the trans-septal dilator and needle. Further studies to prospectively examine the ZFTSP procedure in a randomised, controlled fashion are forthcoming. Through this technique, the possibility of a truly non-fluoroscopic procedure may be readily accessible to many active cardiac catheterisation laboratories.