Søren Zöga Diederichsen, EHRA 2023: NT-proBNP as a tool for atrial fibrillation screening, post hoc analysis of the LOOP study
Recently it has been shown that NT-proBNP may be a useful tool for finding people with an increased risk of atrial fibrillation and stroke. In this touchCARDIO interview, we speak with Dr Søren Zöga Diederichsen (Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark) to discuss the post hoc analysis of the LOOP study.
The abstract entitled ‘Atrial fibrillation screening to prevent stroke in patients with high NT-proBNP – a post-hoc analysis of the randomized LOOP study’ was presented at the European Heart Rhythm Association congress, 16–18, April 2023.
Questions:
- What is known about the role of N-terminal prohormone of brain natriuretic peptide (proBNP) in the pathogenesis of atrial fibrillation? (0:16)
- What are the aims, design, and eligibility criteria of the LOOP study post-hoc analysis? (1:21)
- What were the key findings? (2:03)
- What will be the likely clinical significance of these findings? (3:22)
Disclosures: Søren Zöga Diederichsen is a consultant for Cortrium and Vital Beats, on the advisory board for Acesion Pharma, Bristol Myers Squibb and Pfizer, and is on the speaker’s bureau Bayer, Bristol Myers Squibb and Pfizer.
Support: Interview and filming supported by Touch Medical Media. Interview conducted by Danielle Crosby.
Filmed as a highlight of EHRA 2023
Access more content on atrial fibrillation here
Transcript:
Hi I’m Søren Zoega Diederichsen from University Hospital Copenhagen, Rigshospitalet.
Q. What is known about the role of N-terminal prohormone of brain natriuretic peptide (proBNP) in the pathogenesis of atrial fibrillation?
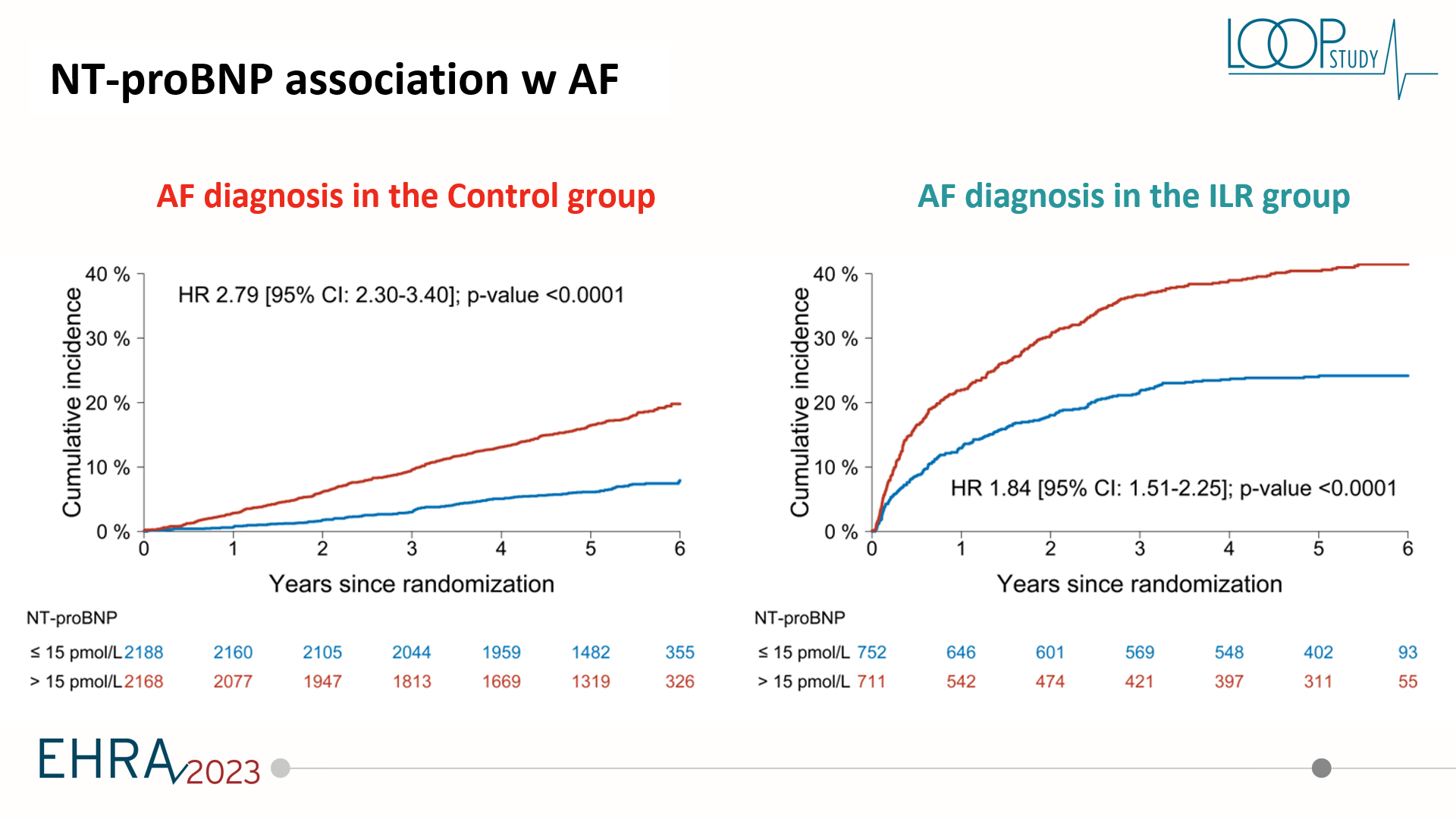
NT-proBNP was originally developed as a marker for heart failure detection and heart failure prognosis. But recently, we are starting to understand that NT-proBNP may be more useful in finding people with an increased risk of atrial fibrillation and stroke. This was investigated in STROKESTOP where they could see that using NT-proBNP in the subgroup of that study could be used to increase the yield of screening. This is also being used in the STROKESTOP 2 trial where the whole screening program is designed, taking into account proBNP, to determine who should have a more intense screening to actually decrease stroke rates. Now in the LOOP study, we also looked at NT-proBNP at baseline from those who are screened using a loop recorder compared with usual care.
Q. What are the aims, design, and eligibility criteria of the LOOP study post hoc analysis?
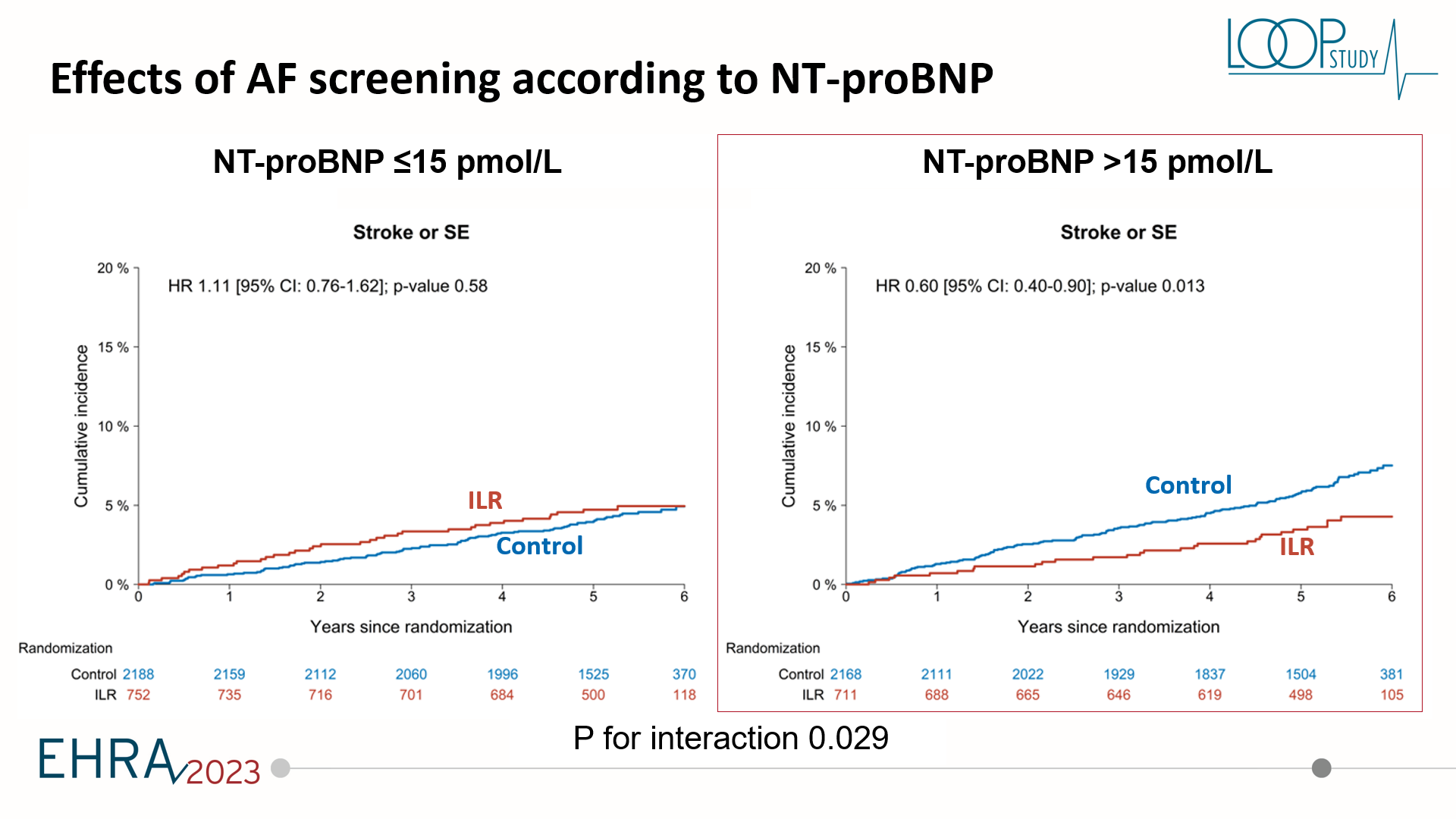
The aim of this post hoc of the LOOP study is to see if baseline proBNP level is associated with an increased screening yield for the loop recorder compared with usual care, and with increased benefit of being diagnosed and being in the screening group. The design was that we took the entire study and we had proBNP measured at baseline on 97% of the study population. Then we looked at the outcomes in groups by proBNP.
Q. What were the key findings?
The key findings of this post hoc analysis was that proBNP was associated with screening yield both in the screening arm, but also associated with increased likelihood of being diagnosed with atrial fibrillation by usual care. ProBNP was not really associated with stroke risk in the whole study population. But very importantly, a higher proBNP was associated with benefit from being screened, whereas a lower proBNP was associated with no signal towards the benefit. This could potentially be important. We looked at proBNP both in the categorial term and as a continuous variable and we suggested a cut-off of about 125 picograms per millilitre or 15 picamole. This could potentially be a useful cut-off as to see these patients above this cut-off could potentially have a benefit from actually being screened for atrial fibrillation. Whereas if you have a lower value, you would not have a benefit even if you were diagnosed.
Q. What will be the likely clinical significance of these findings?
For the clinical significance of these findings, I think we should really keep in mind this was an exploratory study and a post hoc analysis of a large clinical trial. But it is promising that we can use biomarkers to determine who will actually have a benefit from being diagnosed. We know that screening for atrial fibrillation is coming, whether we want it or not. So we can potentially use these biomarkers to increase our own advantage as clinicians on who should actually be monitored a little bit more and who should definitely not worry so much about atrial fibrillation.
So we are enthusiastic about looking forward to the STROKESTOP 2 outcomes, which I think will be reported within the next year.We also want to design other studies for refined screening where we actually, not screen everybody, but maybe choose to screen depending on by instance, biomarkers. And of course, we are going to look a little bit more into this. We are comparing also with other research groups that have used proBNP in a risk assessment for stroke such as the ABC score, another type of stroke assessment as the usual CHA2DS2-VASc score. So right now this is very promising. We should do further studies, but I do not think we should take proBNP in every patient. We have to determine if they should have a screening.
Subtitles and transcript are autogenerated
Additional resources:
Access the published article of the post hoc analysis here