Rivaroxaban is a direct factor Xa inhibitor indicated to reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation (AF), treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE). Furthermore, it is prescribed for risk reduction and prophylaxis for DVT and PE following hip or knee replacement surgery and is one of the most commonly prescribed direct oral anticoagulants (DOACs).1
A substantial discrepancy exists between the recommended dosage and administration and real-world use of DOACs, putting patients at risk of serious thromboembolic events.2–4 Patients taking rivaroxaban are uniquely susceptible to administration errors due to dose-dependent requirements for co-administration with meals. As rivaroxaban has dose-dependent bioavailability, concomitant food recommendations vary with the dose.1,5 The bioavailability of the 10 mg dose is estimated to be 80–100% and is not affected by food, whereas the bioavailability of the 20 mg dose in the fasted state is approximately 66%. Food increases the bioavailability of the rivaroxaban 20 mg dose (mean area under the curve [AUC] and maximum concentration [Cmax] increasing by 39% and 76%, respectively) and also decreases inter-individual variability, which ultimately improves the predictability of the pharmacokinetics.6 Kubitza et al. determined that the fat content of the meal was less important on the bioavailability of rivaroxaban, as they found no differences between a high-fat, high-calorie meal versus a high-carbohydrate meal.6 Thus, taking rivaroxaban in a fasting state or with a small meal could reduce bioavailability and potentially increase the risk of thromboembolic event. The clinical implications of administering rivaroxaban with inconsistent meals or in the fasting state was highlighted in a case report of an individual that experienced recurrent PE due to irregular intake of meals during shift work.7
The recommended dose of rivaroxaban in patients with AF and normal renal function is 20 mg orally, once daily with the evening meal,1 which is how the drug was administered in the pivotal, phase III Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism in Atrial Fibrillation (ROCKET AF) study.1,8 However, it does not appear that adherence to a full meal nor the macronutrient content of such meal was captured in ROCKET AF.8 The dose for patients with a creatinine clearance 15–50 ml/min is 15 mg.1 Dosages for treatment of DVT, PE or reduction in risk of recurrent venous thromboembolisms are 15 mg orally twice daily with food for the first 21 days, followed by 20 mg once daily with food. Dosages for prevention of DVT after hip or knee replacement surgery is 10 mg once daily with or without food.1
In addition to errors in administration with appropriate meals, rivaroxaban is not always dosed correctly, especially in patients with renal dysfunction. A recent analysis assessed DOAC dosing patterns and associated outcomes in 14,865 patients with AF and renal dysfunction.3 In patients with a renal indication for a reduced dose of rivaroxaban, 41.3% received standard doses. In patients with no renal indication for dose reduction, 15.1% of patients were underdosed. In those patients with a renal indication for dose reduction, the event rate per 100 person-years of ischaemic stroke or systemic embolism was 1.18 (0.26–11.45) for those on the reduced dose and 2.84 (1.19–8.47) for those on standard dosing. In the same cohort, the rate of major bleeding was 5.88 (3.19–11.94) for those on reduced dosing and 10.98 (7.01–18.05) for those on standard dosing, suggesting that overdosing nearly doubled the risk of bleeding without similar reduction in the risk of stroke. An increased risk of cerebral vascular accident was found in patients with normal renal function that were ‘underdosed’ in apixaban-treated patients, but not dabigatran or rivaroxaban.3 Not only can inappropriate prescribing affect clinical outcomes but patient adherence can also be detrimental. In another recent study, it was found that non-persistence with both rivaroxaban and dabigatran was associated with increased risk of cerebral vascular accident and or death.4
This retrospective study documents provider prescribing and real-world patient adherence to dietary requirements during self-administration of rivaroxaban for AF.
Methods
This retrospective study was conducted at Bryan Health Medical Center in Lincoln, Nebraska between January 2014 and January 2017 in 116 consecutive, unique individuals. The protocol was reviewed and deemed exempt by the Creighton University Institutional Review Board. Patients were included if they were seen in cardiology consultation for AF and were already taking rivaroxaban prior to evaluation.
Data collected from each patient included prescribed dose, renal function (current and at the time of rivaroxaban initiation), prescriber specialty and patient adherence. Prescriber specialty was the specialty of the provider that initiated the prescription prior to the cardiology consult. Renal function was calculated using the Cockcroft-Gault equation using actual body weight. If the patient’s actual body weight was greater than 20% of ideal, then adjusted body weight was used. A patient was considered dosed inappropriately using their renal function at the time of prescribing. Patients were considered non-adherent if they reported missing any doses, if they were administering during a fasting state or administering only with a small meal or snack. All patient adherence data was self-reported. The same investigator, a cardiologist, assessed whether or not the patient was taking with an adequate meal by asking the patient if they were taking with a meal, which meal and what the meal consisted of.
Categorical variables are presented as frequency and percent. Associations between professional specialty and the correct dosing and administration were assessed using the Chi-Square test. All statistical analysis was performed in IBM SPSS Statistics for Windows, Version 24.0 (Armonk, NY: IBM Corp.).
Results
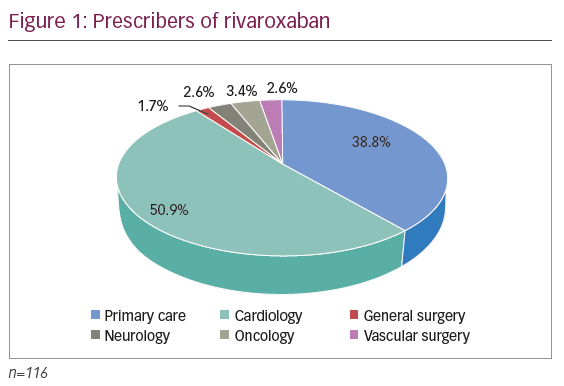
Figure 1 indicates that the majority of prescriptions for rivaroxaban in this sample were ordered by cardiologists (59, 50.9%), followed by primary care physicians ([PCP] 45, 38.8%), oncologists (4, 3.4%), neurologists (3, 2.6%), vascular surgeons (3, 2.6%) and general surgeons (2, 1.7%).
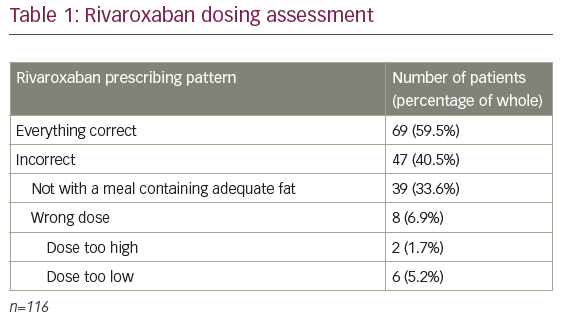
Of the 116 patients assessed, 69 (59.5%) were taking the correct dose based on indication and renal function and taking at the correct time with an adequate meal (see Table 1). Of the 47 (40.5%) taking rivaroxaban incorrectly, 39 (33.6%) were not administered with an adequate meal and 8 (6.9%) were not prescribed the correct dose. Of the incorrectly dosed patients, 2 (1.7%) were too high and 6 (5.2%) were too low. All patients reported that they had not missed any doses.
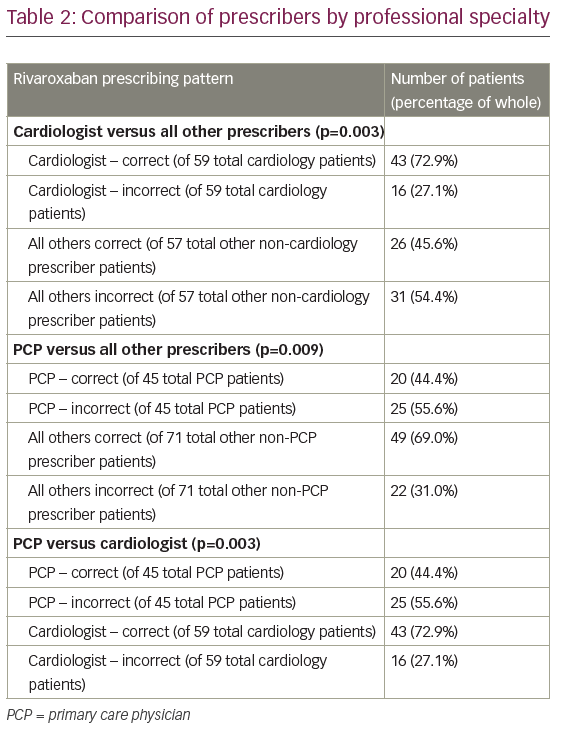
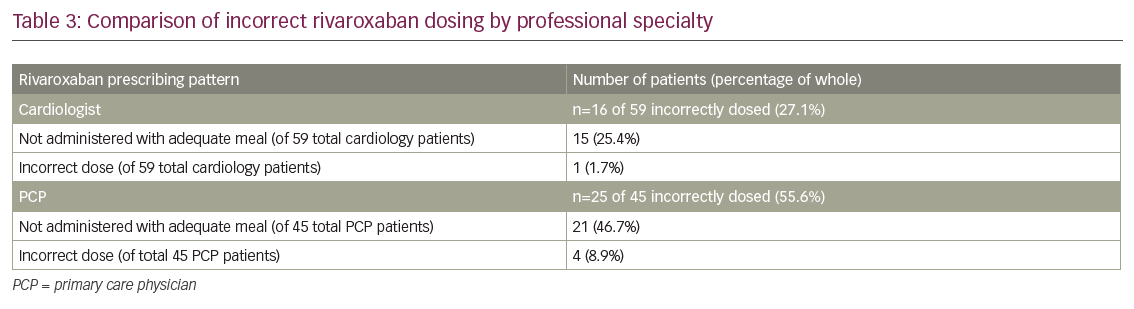
Table 2 compares the prescribing pattern of rivaroxaban by professional specialty. Compared with all other prescribers, patients were most likely to be taking the correct dose with correct administration when prescribed by cardiologists (72.9% correct versus 45.6% correct, respectively; p=0.003). Patients were least likely to be taking the correct dose with correct administration when prescribed by PCP (44.4% correct versus 69.0% correct, respectively; p=0.009). It should be noted that of the 25 patients that were prescribed rivaroxaban by a PCP and taking it incorrectly, only 4 (8.9%) were dosed incorrectly by the prescriber (see Table 3). However, 21 (46.7%) of patients taking rivaroxaban prescribed by a PCP were not administered with an adequate meal.
Discussion
Inappropriate prescribing, non-adherence and inappropriateadministration of anticoagulation can have devastating consequences. This study demonstrated a gap in patient awareness of proper administration of rivaroxaban with an adequate meal in that 33.6% of patients were not taking with a meal. This is similar to the 32.3% recently reported by Tellor et al.9 During this process, many patients anecdotally reported no knowledge of the requirement to take with an adequate meal. Patients under a cardiologist’s care were most likely to be taking rivaroxaban correctly and this may be due to increased exposure of this specialty to continuing education regarding prescribing of DOACs. Alternatively, the advantage may be due to a team-based approach to care often utilised in cardiology practice groups. Both inpatient and outpatient care of AF patients is often multidisciplinary, allowing for collaborative and shared responsibility for patient education amongst cardiologists, mid-level providers, nurses and pharmacists.
Conversely, of the 45 patients prescribed rivaroxaban by a PCP, 21 (46.7%) were not administered with an adequate meal. These results highlight the opportunity for community pharmacists to play a role in closing the gap in patient education as it relates to the dosing and administration of rivaroxaban. Community pharmacists are often the last healthcare providers in contact with the patient before outpatient medication administration. Survey data to assess the rivaroxaban patient education practices from various providers and also community pharmacists are warranted to better explain these findings.
Of the eight (6.9%) patients prescribed the incorrect rivaroxaban dose, the majority (six patients) were underdosed. While underdosing has the potential to increase risk of cerebral vascular accident, overdosing appears to have nearly double the adverse bleeding outcomes without reduction in risk of stroke.3 Aside from renal function, clinicians must also take into consideration bleeding risks, drug interactions, anticipated changes in renal function and patient fragility.3 Thus, there is often relevant justification for a prescriber to deviate from a recommended renal-based dose of a DOAC. The 6.9% rate of ‘incorrectly’ prescribed doses of rivaroxaban for AF at our institution is much lower than the 35.4% recently reported by Tellor et al. at another tertiary care community hospital in the Midwest.9
Limitations
One limitation of this study is the fact that it is small, conducted at a single institution, and only assessed one indication (AF) for rivaroxaban. Thus, caution should be utilised when extrapolating these results to the general population or for other DOACs or indications. The retrospective nature of this study also forced investigators to rely on prescriber documentation thus leaving room for bias. As previously mentioned, the number of prescribers for certain disciplines was small and therefore there was no meaningful way to compare their prescribing habits independently. The investigators also relied on patient reporting for adherence and meals. For simplicity, it was only recorded if the patient took the medication with a meal or no meal/snack. Macronutrient content was not assessed. Whether or not the patients received education was also not captured; therefore, conclusions based on counselling practices need to be confirmed with further research.
Finally, because rivaroxaban is a once-daily DOAC for AF, which does not require monitoring like vitamin K antagonist and is generally well tolerated, it has been associated with greater adherence and persistence compared with other anticoagulants.10,11 Consequently, prescribers may be more likely to prescribe rivaroxaban patients with documented historical non-adherence. Therefore, non-adherence to the dietary requirements observed in this study may be more attributable to patient characteristics as opposed to lack of patient education by the healthcare providers.
Conclusions
Though patients prescribed rivaroxaban by a cardiologist in this study were more likely to have received education regarding correct administration of rivaroxaban, there is clearly an education gap that has been supported by other studies.9 This highlights the importance of formal systematic education for all patients prescribed rivaroxaban and other DOACs, in the hospital, office and community settings, including information regarding co-administration with a meal. Future studies are also warranted to explore the impact of non-adherence to rivaroxaban dietary requirements on clinical outcomes.