Percutaneous coronary intervention (PCI) has evolved considerably in both technique and technology since Andreas Grüntzig first intervened on a focal, proximal lesion in the left anterior descending artery in 1977.1 While this has resulted in increasingly complex anatomical subsets being treated, contemporary trials of PCI in patients with stable coronary artery disease (CAD) have continued to exclude patients with severe left ventricular systolic dysfunction (LVSD), despite CAD being the most common cause of heart failure with reduced ejection fraction.2 Prior observations have suggested that revascularizing viable but dysfunctional ‘hibernating’ myocardium would improve left ventricular function, with positive effects in reducing heart failure decompensation and death.3 The lack of randomized evidence to support revascularization with PCI in this context has, however, led to divergent practice and guideline recommendations, with American guidelines not providing a recommendation,4 while European guidelines give a class IIa recommendation.5
The REVIVED-BCIS2 trial (Study of efficacy and safety of percutaneous coronary intervention to improve survival in heart failure; ClinicalTrials.gov identifier: NCT01920048) set out to answer the following question: does revascularization with PCI in addition to optimal medical therapy (OMT) in patients with ischaemic LVSD and stable CAD reduce the occurrence of all-cause death or hospitalization for heart failure compared with OMT alone?6 To capture patients most likely to benefit from PCI, participants were required to have a left ventricular ejection fraction of ≤35%, extensive CAD (British Cardiovascular Intervention Society jeopardy score ≥6) and at least four dysfunctional myocardial segments viable and amenable to percutaneous revascularization. The trial recruited 700 participants across 40 centres in the UK with a median follow-up of 3.4 years. Although it is possible that some patients with non-ischaemic LVSD or dual pathology had been recruited,7 it is important to note that 326 patients, representing 16% of the excluded patients following screening, were not recruited, as left ventricular impairment in these patients was felt to be disproportionate to the level of coronary disease. Moreover, in 491 patients, cardiac magnetic resonance imaging was the viability testing method used and corroborated that the amount of subendocardial late-gadolinium scar was consistent with an ischaemic cause of LVSD.8 The main finding of the study was that, at 2 years following randomization, PCI in addition to OMT did not reduce the occurrence of the primary outcome compared with OMT alone (37.2% versus 38.0%; hazard ratio 0.99, 95% confidence interval 0.78–1.27; p=0.96).6 While the primary focus is on PCI failing to improve outcomes above OMT alone, the results also highlight the poor outcomes of this population; almost one-third of participants had died at 2 years, highlighting the need to continue improving the therapeutic options.
A key pre-specified secondary outcome measure was the change in left ventricular ejection fraction; PCI failed to improve the ejection fraction beyond the improvement seen with OMT alone.6 This finding calls into question the theory of hibernating myocardium or at least suggests that OMT has equivalent efficacy to revascularization in improving contractility (when deaths were excluded, the mean improvement in ejection fraction was around 6% in both groups). These findings are similar to the Surgical Treatment for Ischemic Heart Failure (STICH) viability sub-study (Comparison of surgical and medical treatment for congestive heart failure and coronary artery disease; ClinicalTrials.gov Identifier: NCT00023595), where only patients with viable myocardium improved their ejection fraction, irrespective of whether revascularization took place.9 A second pre-specified outcome was change in quality of life measured by the Kansas City cardiomyopathy questionnaire. This outcome did show a benefit in the PCI group at 6 months; however, by 24 months, Kansas City cardiomyopathy questionnaire scores in the OMT alone group improved so that there was no longer a significant difference.6 We cannot exclude the possibility that the open-label nature of the REVIVED-BCIS2 trial may have contributed to the initial improvement seen in the PCI group and that, given the lack of difference at 24 months, caution must be applied when considering exposing a patient to the risks of PCI with the aim of improving quality of life alone.
The findings of the REVIVED-BCIS2 trial contrast with those of the STICH trial, which investigated the benefit of surgical revascularization with coronary artery bypass grafting (CABG) for ischaemic LVSD. In the STICH trial, CABG provided an incremental mortality benefit over OMT at long-term follow-up.10,11 Nonetheless, several important considerations must be taken into account when comparing these two studies. Firstly, participants in the REVIVED-BCIS2 trial were older (the mean age was 70 years compared with 60 years in the STICH trial), which is an important distinction, as most of the observed benefit in the STICH trial occurred in patients <60 years of age;11 this finding also highlights the particular need for improved therapies in most patients with ischaemic LVSD who are not candidates for CABG. Secondly, OMT has improved since the STICH trial completed recruitment in 2007, so one may speculate that the benefits observed in this trial may not be apparent were the study to be repeated now. Most importantly, CABG represents a fundamentally different method of revascularization, where all myocardial territories will generally be revascularized regardless of viability status, a factor that may have a significant impact on subsequent spontaneous myocardial infarction and ventricular arrhythmias. The results of the REVIVED-BCIS2 arrhythmia sub-study are awaited to support this theory.
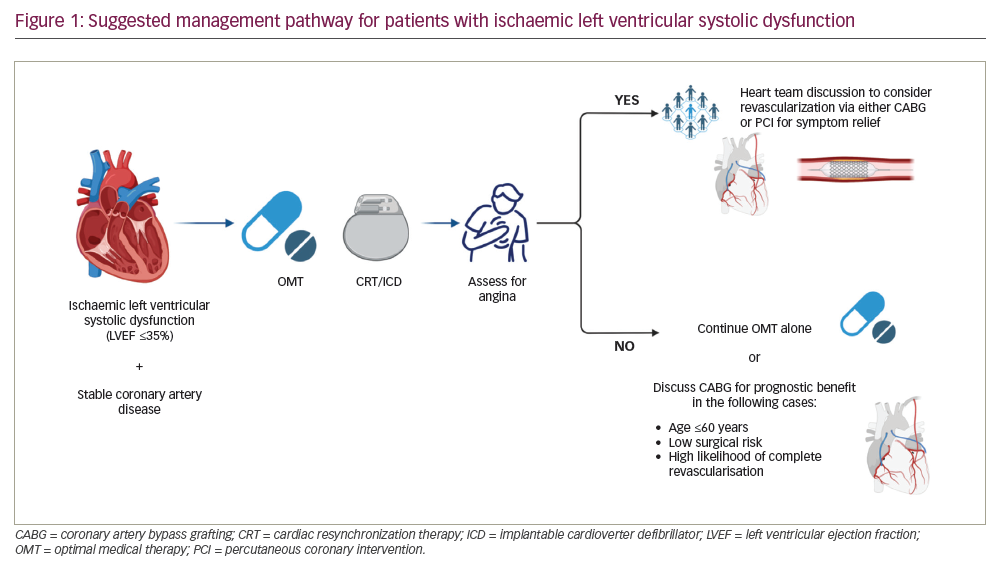
With the results of these two landmark studies, we propose a management pathway for patients with stable CAD and ischaemic LVSD (Figure 1). Management should be individualized, that is, based on patient characteristics, comorbidities and the opinion of a multidisciplinary team. All patients should initially be treated with OMT and assessed for cardiac resynchronization therapy with or without cardiac defibrillator, as per guideline recommendations.12 Following this step, the patient should be carefully evaluated for symptoms of angina; if present, revascularization may be considered for symptom relief via either CABG or PCI since no excess risk was observed early after PCI.6 In the absence of symptoms of angina, PCI plays no role in managing these patients, while CABG may be considered in younger patients at relatively low operative risk and where complete revascularization is likely, given the evidence of prognostic benefit seen in STICH.11
Conclusions
In summary, the REVIVED-BCIS2 trial found that PCI in addition to OMT offers no prognostic benefit compared with OMT alone in patients with ischaemic LVSD and stable CAD; therefore, it should only be considered in patients with an acute coronary syndrome or limiting angina. Given the observed differences between patients in the REVIVED-BCIS2 trial and those in the STICH trial, randomized comparisons of CABG and PCI in surgically eligible patients would add significantly to the current evidence, with protocols in development by an international network of clinical triallists.