Reperfusion of infarct-related artery (IRA) in acute ST-elevation myocardial infarction (STEMI) is the procedure with the most clinical benefit in interventional cardiology. However, in around 50% of patients presenting with STEMI, other significant lesions are present, a finding which is associated to a worse prognosis.1,2 Most physicians performing primary percutaneous coronary intervention (PCI) have been trained according to a ‘keep it as simple as possible’ teaching, limiting the procedure to stent implantation on the culprit lesion only.3–5 This treatment paradigm was based on several considerations:
- acute myocardial infarction is associated with an inflammatory state that might predispose to acute stent thrombosis;
- platelet inhibition is often not complete because antiplatelet drugs have not reached their full level of activity (which can be partially reconsidered nowadays, as more potent and rapid antithrombotic drugs have become available);
- should a complication occur on a non-culprit vessel, this might cause an extensive ischaemic area and jeopardise haemodynamic stability;
- vasoconstriction is usually present during acute myocardial infarction, which might cause overestimation of other lesions and inadequate vessel sizing;6 and
- renal function and other comorbidities (e.g. significant anaemia) are often not known at the time of primary PCI, when blood tests are generally not available; this concept must be kept in mind, as the population we are currently treating is getting older and more complex.
On the other hand, one could argue that instability might not be confined to the culprit lesion, but it can be related to a widespread process involving the whole coronary tree, so that other plaques might be predisposed to acute events.7 Indeed, several studies have questioned the traditional teaching of treating IRA only during primary PCI.8–14
In this review article, currently available trials and meta-analyses comparing IRA-only and multivessel revascularisation for patients with acute myocardial infarction and multivessel coronary artery disease are reviewed. A search was performed for the following keywords in the PubMed database: ‘percutaneous coronary intervention’, ‘myocardial infarction’ and ‘multivessel’. We specifically focused on selection criteria and timing of revascularisation of non-culprit lesions. Five large randomised trials comparing IRA-only versus complete revascularisation were identified; details of these trials are summarised in Table 1.
PRAMI trial
In the PRAMI (Preventive Angioplasty in Acute Myocardial Infarction) trial, 465 patients were randomised to PCI on all lesions ≥50% during index procedure versus revascularisation of IRA-only.10 In patients undergoing IRA-only PCI, subsequent stenting of other lesions was discouraged unless refractory angina with objective evidence of inducible ischaemia was present. The study was prematurely stopped because of significantly lower incidence of the primary endpoint (death, myocardial infarction or refractory angina) in the multivessel PCI group; the comparison maintained statistical significance when the two hard endpoints (death and nonfatal myocardial infarction) were analysed.
CvLPRIT trial
The CvLPRIT (Complete versus Lesion-Only Primary PCI) trial confirmed the benefit of complete revascularisation in 296 patients with STEMI with multivessel disease.11 The primary endpoint (a composite of all-cause death, recurrent myocardial infarction, heart failure, and ischaemia-driven revascularisation within 12 months) occurred in 10.0% in the complete revascularisation group versus 21.2% in the IRA-only arm (hazard ratio [HR]: 0.45; 95% confidence interval [CI]: 0.24–0.84; p=0.009).11 In the complete revascularisation group, multivessel PCI was performed at the time of primary PCI in two-thirds of patients; in the remaining cases, based on operators’ decision, revascularisation of non-culprit lesions was postponed to a staged procedure within the same hospitalisation. Patients treated with immediate multivessel PCI tended to have better results compared to staged procedure, although statistical significance was not reached and a selection bias can be hypothesised.
DANAMI-3-PRIMULTI trial
Angiographic appearance of non-culprit lesions might lead to overestimation of lesion severity and therefore to unnecessary stenting. However, there are theoretical concerns that fractional flow reserve (FFR) in acute myocardial infarction could not be reliable due to alterations in microcirculatory function and coronary flow.15 Ntalianis et al. validated the use of physiological assessment during myocardial infarction by performing FFR in 112 non-culprit lesions at time of primary PCI; the authors repeated the measurements at 1-month follow up, showing that FFR values remained consistent.16
The DANAMI-3-PRIMULTI (Complete Revascularisation versus Treatment of the Culprit Lesion Only in Patients with ST-Segment Elevation Myocardial Infarction and Multivessel Disease) study included 627 patients after successful IRA-only PCI; a strategy of FFR-guided complete revascularisation was compared to no further invasive treatment.12 Non-IRA PCI was performed before discharge. The primary endpoint of all-cause mortality, nonfatal myocardial infarction and ischaemia-driven revascularisation of lesions occurred in 13% of patients who had complete revascularisation versus 22% in the IRA-only PCI arm (HR: 0.56; 95% CI: 0.38–0.83; p=0.004). The benefit was, however, driven by a reduction in repeat revascularisation, an outcome that might be biased by the awareness that patients have one or more stenoses left untreated. Of note, 31% of non-IRA lesions evaluated with FFR were haemodynamically non-critical.
Compare-Acute trial
The Compare-Acute trial enrolled 885 patients with STEMI and multivessel disease; after IRA PCI, FFR was performed on all lesions of >50% and patients were randomised 1:2 to complete revascularisation or medical therapy.13 The incidence of primary endpoint (death, myocardial infarction, revascularisation or stroke) was reduced in the complete revascularisation arm (HR: 0.35; 95% CI: 0.22–0.55; p<0.001); again, the benefit was driven mainly by a reduction in the need for revascularisation at a later time point. In the complete revascularisation group, PCI of non-IRA lesions was performed during primary PCI procedure in 83% of cases, but could be deferred and staged within index hospitalisation, as in DANAMI-3 PRIMULTI.12 In the IRA-only group, subsequent management was left to the referring cardiologist’s decision: in 59 patients in this arm elective PCI on non-IRA lesions was performed within 45 days after the index procedure, which was not counted as an adverse event. Another analysis of 36-month results of this trial confirmed the clinical benefit of FFR-guided complete revascularisation, which also reduced net healthcare-related costs.17
COMPLETE trial
The recently published COMPLETE (Complete versus Culprit-Only Revascularization Strategies to Treat Multivessel Disease after Early PCI for STEMI) trial is the largest randomised study on this topic, enrolling 4,041 patients randomised 1:1 to culprit-lesion only PCI or complete, staged revascularisation.14 Non-culprit lesions were considered significant either if ≥70% or if ≥50% with FFR ≤0.80. In the complete revascularisation arm, according to operator’s choice, non-culprit lesion treatment could be performed either during index hospitalisation or at a later admission, within 45 days. The primary endpoint of death or myocardial infarction occurred more frequently in the culprit lesion-only PCI group (10.5% versus 7.8%; HR: 0.74; 95% CI: 0.60–0.91; p=0.004) and the benefit of complete revascularisation was consistent among patients undergoing complete revascularisation during the same admission or at a later time.
Meta-analyses
Elgendy et al. performed a meta-analysis comparing four different strategies:18 complete revascularisation at the time of index procedure, staged revascularisation before discharge, staged revascularisation at a second admission, and IRA-only revascularisation. Data on 2,285 patients from 10 trials were included (not comprising results from Compare-Acute and COMPLETE trials). Compared with a culprit-only approach, complete revascularisation (either immediate or staged) was associated with a lower risk of major adverse cardiac events due to a reduction in urgent revascularisation procedures; the rate of mortality or spontaneous myocardial infarction was comparable. No major differences in outcomes were found between immediate or staged complete revascularisation.
Another meta-analysis, published by Tarantini et al. in 2016, compared three different approaches: culprit-only PCI, immediate complete revascularisation, and staged PCI (either during the same admission or after discharge).19 The authors concluded that staged multivessel revascularisation had superior outcomes in terms of mortality rate when compared both to immediate multivessel revascularisation and culprit-only PCI. The benefit of staged PCI is particularly apparent in trials enrolling a high proportion of three-vessel disease and patients with diabetes.
In the same year, a critical review on this topic identified 25 meta-analyses comparing multivessel versus culprit-only PCI in STEMI; of those, 6 showed increased mortality after multivessel PCI, 10 were neutral and 9 found decreased mortality.20 The profound difference in results mainly depends on which studies were included in each paper and on statistical methodology. Bainey and colleagues recently published a meta-analysis on over 7,000 patients from 10 studies; complete revascularisation was associated with reduced cardiovascular mortality. The benefit was unchanged with FFR or angiography-guided strategies; also, no differences were found between an immediate or staged approach.21
Multivessel disease in ST-elevation myocardial infarction and cardiogenic shock
While in haemodynamically stable patients evidence moved from culprit-only to multivessel PCI, somewhat the opposite has occurred for patients presenting with acute myocardial infarction and cardiogenic shock. Indeed, the CULPRIT-SHOCK trial showed that a strategy of culprit-only PCI, compared with multivessel primary PCI (on all lesions ≥70%), was associated with a significant reduction in a composite of death and severe renal failure (45.9% versus 55.4%; relative risk 0.83; 95% CI: 0.71–0.96; p=0.01), mainly due to a mortality benefit.22 Of note, subsequent staged revascularisation was encouraged in the culprit-only group, and performed in 17.7% of patients; a crossover rate of 12.5% occurred from culprit-only to immediate multivessel PCI, based on the operator’s decision. According to this trial, revascularisation of non-culprit lesions during acute myocardial infarction presenting with cardiogenic shock was downgraded as a class III, level B indication in European Society of Cardiology guidelines.23
Discussion
Taken together, available randomised data consistently suggest that, in haemodynamically stable patients, complete revascularisation is superior to culprit-artery revascularisation only. There are, however, two issues that are not completely clarified by the available evidence; these are, which non-culprit lesions should be revascularised and when should this be performed? Regarding the optimal timing of revascularisation on non-IRA lesions, an immediate multivessel procedure has the advantage of avoiding a second catheterisation, and could be preferable for patient comfort, risk of access-site bleeding and cost issues. However, deferring revascularisation of non-culprit lesions to a second procedure can give the advantages of overcoming the inflammation and vasoconstriction associated with the acute phase, it also allows for stable platelet inhibition and reduces the amount of contrast during primary PCI, overcoming the concerns that traditionally discouraged multivessel revascularisation in STEMI.24
Should the operator decide on a staged procedure, it might be deferred to a second, elective admission, or if it could be performed during the same hospitalisation. The 2018 Guidelines on myocardial revascularisation from the European Society of Cardiology, based on a study design of the available trials, concluded that routine non-IRA revascularisation should be performed before hospital discharge, with a class IIa grade A recommendation.23 However, in the COMPLETE trial, a significant proportion of multivessel PCIs were deferred to a second admission, with no apparent differences compared to in-hospital staged procedures.14 Moreover, data from a yet unpublished large registry suggest that the optimal window for revascularisation of non-culprit lesions might be between 10 and 28 days after primary PCI (Zhang et al., unpublished data).
We do believe that, when firm evidence from high-quality trials is lacking, the decision should be based on clinical judgement and individual physician’s experience. Indeed, sophisticated statistical analyses, as pointed out in a paper by Bates et al.,20 can lead to support disparate conclusions, and mixing up results from different trials trying to identify a single approach for every situation inevitably blurs the characteristics of specific clinical settings.
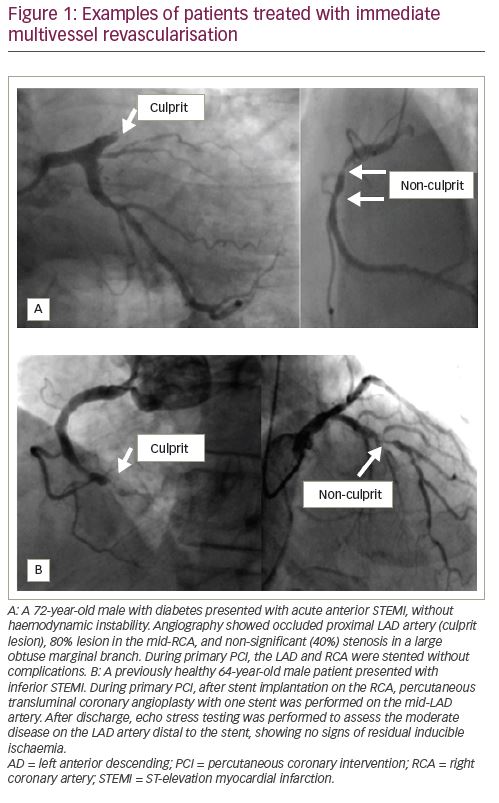
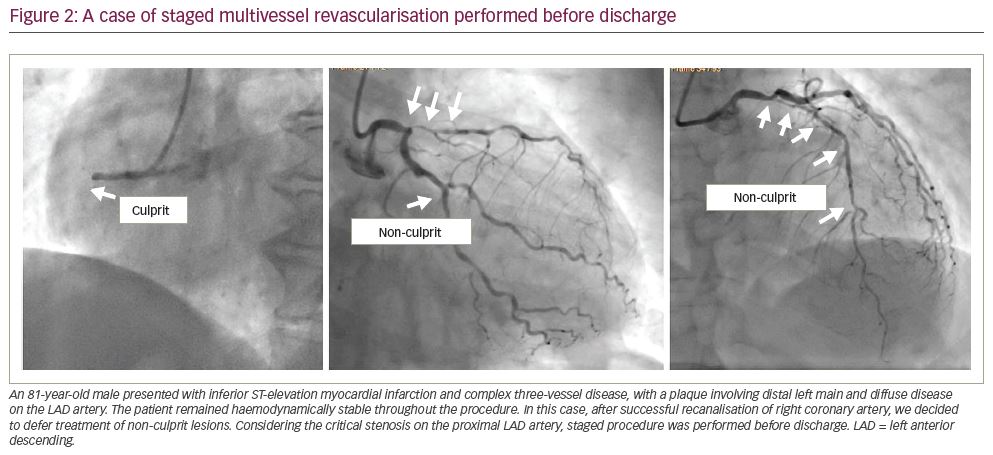
Available evidence clearly suggests that complete revascularisation should be preferred to IRA-only PCI; if the residual disease can be easily treated during primary PCI (i.e., one or two focal lesions on non-IRA vessel) and the patient is haemodynamically stable, this can be safely performed (as in the two examples shown in
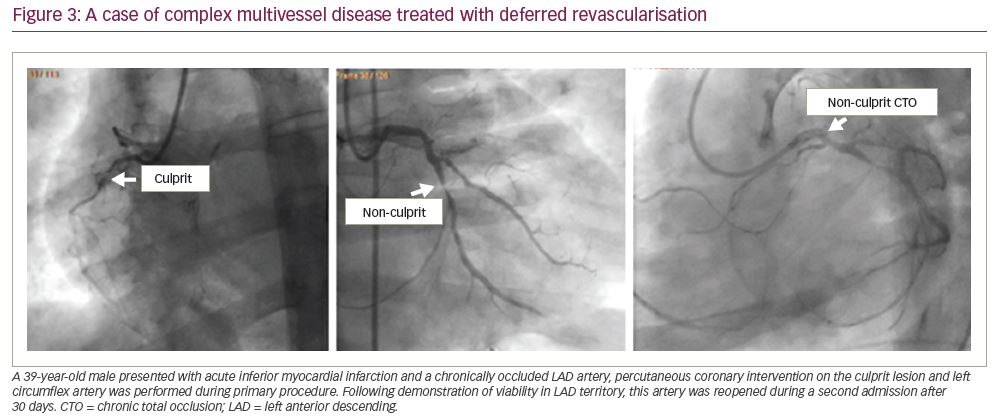
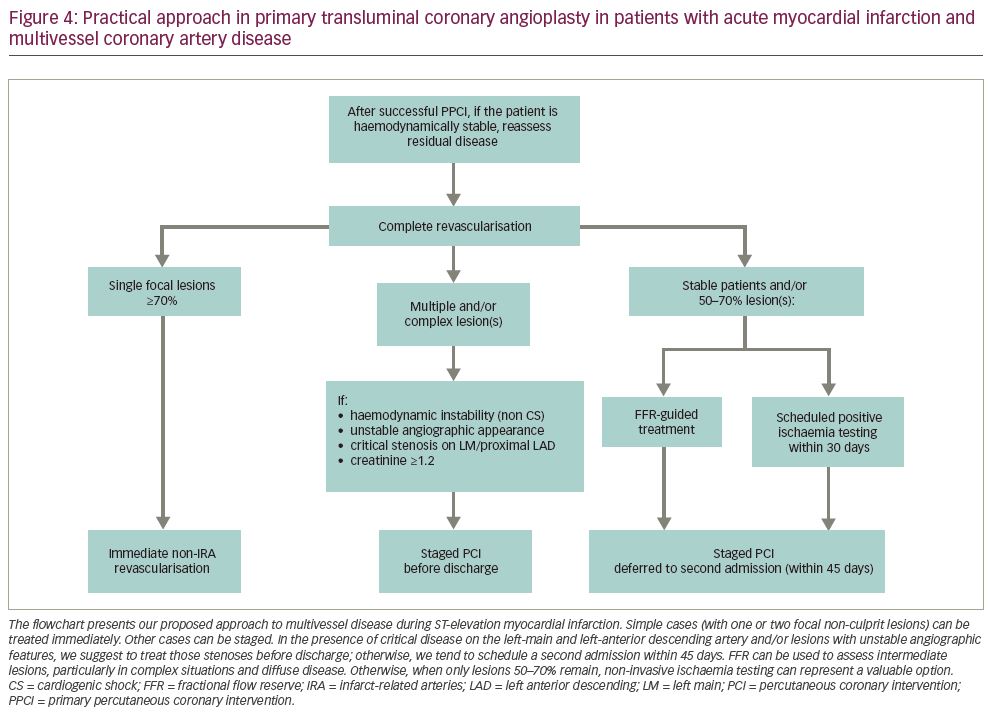
Figure 1). If instead, treatment of non-culprit stenoses requires a complex procedure (e.g. bifurcations, diffuse disease, chronic total occlusions, calcific plaques), it is advisable to schedule a second intervention (Figure 2). In our experience, as confirmed by results from COMPLETE trial, there is no significant risk in deferring revascularisation to a second admission, unless residual lesions have unstable angiographic features and/or involve the left main or proximal left anterior descending artery (Figure 3). The timing of staged PCI (before discharge or at a second admission) can, therefore, be left up to the operator’s choice, taking into account angiographic appearance and location of lesions, renal function, risk of bleeding, local cost issues and the patient’s preferences. Our default approach is summarised in the flowchart presented in Figure 4.
The other unclear aspect is how to define which non-culprit lesions should be treated. Recent trials used different approaches, ranging from PCI on all stenoses ≥50% to routine FFR evaluation of all non-culprit lesions. In many physicians’ minds, once the acute lesion has been fixed with primary PCI, the residual coronary artery disease can be assessed and treated with the same approach we use for chronic coronary syndromes, which is the concept behind the traditional approach of IRA-only revascularisation.
The results from the above cited studies suggest that this assumption is not completely correct, probably because the process leading to clinical instability is not confined to culprit lesions only. Indeed, while recent data have questioned the utility of percutaneous revascularisation over medical therapy in stable angina,25,26 data from patients with acute myocardial infarction consistently show that complete revascularisation is associated with a reduced risk of hard endpoints. This reassures us that PCI has a beneficial effect on mortality and myocardial infarction, provided that it is performed in the appropriate clinical setting. Ideally, we should start to give more consideration to clinical presentation rather than percent stenosis. The same stenosis probably has very different prognostic implications (and therefore a different appropriate treatment) if it is discovered during primary PCI as a non-culprit lesion, or if it causes stable angina, or if it is ‘incidentally’ found after screening tests.
The DANAMI-3 PRIMULTI and Compare-Acute trials have shown that FFR guidance leads to underestimation of a significant proportion of lesions compared with angiographic evaluation, thereby reducing the need for stenting. However, we cannot exclude that part of the clinical benefit observed in the PRAMI and CvLPRIT trials might be due to treatment of lesions that were not currently causing significant ischaemia (and therefore could have been FFR-negative), but were unstable due to widespread inflammation. Indeed, intravascular ultrasound studies have shown that, compared with chronic coronary disease, in acute coronary syndromes, the incidence of unstable features is higher also in non-culprit lesions.27–29 Often, these lesions appear only moderately stenotic at angiography, in part due to positive vessel remodelling. This is consistent with the previous observation, reported over many years, that the extent of luminal narrowing is a poor predictor of subsequent acute events.30–32 On the other hand, intravascular ultrasound studies have failed to demonstrate the power of high-risk features in predicting future clinical events.33 As reported above, in a large meta-analysis, FFR-guided PCI, compared to angio-guided revascularisation, while associated with lower number of implanted stents, resulted equivalent in terms of event rate.21
Based on currently available evidence, the approach adopted in the COMPLETE trial appears sensible: PCI for lesions ≥70% and moderate stenoses with FFR ≤0.80.14 Considering the high cost of FFR, we tend, instead, to perform non-invasive ischaemia testing – within 30 days from index hospitalisation – in case of moderate residual lesions, particularly if they are located on non-prognostic segments.
Another unclear issue is whether the benefit of complete revascularisation after STEMI also applies to chronic total occlusions. Indeed, in the above-mentioned trials, chronic total occlusions were either excluded or present in a very small proportion of patients. One randomised trial, performed on 302 patients, has, however, failed to show a benefit of chronic total occlusion recanalisation after STEMI in terms of left ventricular ejection fraction or end-diastolic volume.34
Conclusions
Based on currently available data, we can state that, in haemodynamically stable patients presenting with acute myocardial infarction and multivessel disease, complete revascularisation should be considered the gold standard approach. In addition, non-IRA revascularisation can be safely deferred (either during index hospitalisation or scheduling a new admission within 45 days), particularly if a complex procedure is anticipated. Furthermore, FFR, instant wave-free ratio or non-invasive provocative tests are recommended for assessment of intermediate lesions.