Background: In the response to the COVID-19 pandemic the accepted arbitrary 12 month follow up of pacemaker patients was called into question. With the inability of seeing patients face to face service delivery was altered to ensure that hospital visits were kept to a minimum whilst maintaining patient safety and care. Appointments were deferred as considered appropriate. A variation in practice between centres regarding follow-up appointment schedules pre and “post” pandemic exists. The aim was to assess the appropriateness and safety of extending bradycardia pacemaker follow-up durations to 18 months, from a yearly review, in stable patients.
Methods: This was a retrospective service evaluation in sequential patients attending pacemaker follow-up before and after the change from routine 12 month appointments to extended appointment duration as a response to the COVID-19 pandemic at Leeds Teaching Hospital NHS Trust (LTHT). Data was collected from all patients who met the inclusion criteria of follow up of 12 months in the pre COVID-19 group (4th November 2019- 3rd December 2019) and compared to extended follow up >18 months in the post COVID-19 group (9th March 2021- 26th April 2021). Clinically relevant follow up data was obtained from Trust databases with safety data and clinical outcomes evaluated.
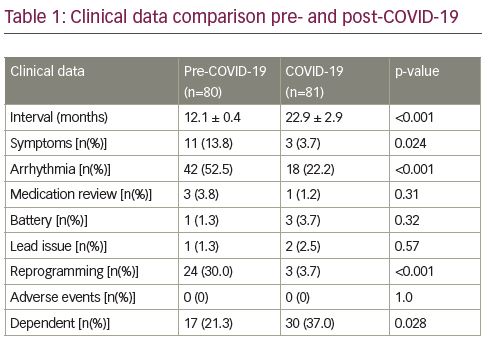
Results: A total of 161 patients met all the inclusion criteria and were assessed. Patients were excluded from the dataset if they had a follow up pacemaker appointment of <12 months. Patients were grouped according to pre COVID-19 (n=81) with an average follow up of 12.1 months ± 0.4 and post COVID-19 (n=80) with an average follow up 22.9 months ± 2.9. Data showed a significant reduction in reported symptoms (13.8% vs 3.7%; p=0.24), arrhythmia burden (52% vs 22.2%; p=<0.001) and reprogramming requirements (30% vs 3.7%; p=<0.001) in the post COVID-19 extended follow up group compared to the pre COVID-19 annual review group. In addition, there was no difference between groups for requirement for medication review, incidence of premature battery depletion, lead issues or adverse events requiring intervention. Lastly, there was a significant higher proportion of patients that had no underlying R-wave in the post COVID-19 extended follow up group (37% vs 21.3%; p 0.028) compared to the pre COVID-19 annual review group (Table 1).
Conclusion: To our knowledge, this is the first service evaluation comparing the clinical outcomes and safety data of extending for stable patients. This service evaluation has demonstrated that in pacemaker dependent patients, that are seen at 18 month appointment intervals there is a reduction in arrhythmia burden and reprogramming requirements. The significant reduction in symptoms reported post COVID-19 could be attributed to the change in physiologist performing the checks in addition to lack of documentation of symptoms reported. This service evaluation has demonstrated that 18 month appointments could be used for all patients and has no detrimental effect to patient safety or care.