Background: Despite the millions of people infected by SARSCoV-2 worldwide, there have only been few reports of cardiac device implantations in such subjects.
Purpose: We report a series of patients with SARS-CoV-2 who needed a permanent pacemaker.
Methods: Study of a single public hospital population in Northern Italy, entirely converted to a COVID-19-only facility during the SARS-CoV-2 pandemic. Data retrospectively collected from electronic medical history. We analysed the clinical profiles of patients implanted with pacemakers, the procedural safety, and the follow-up data.
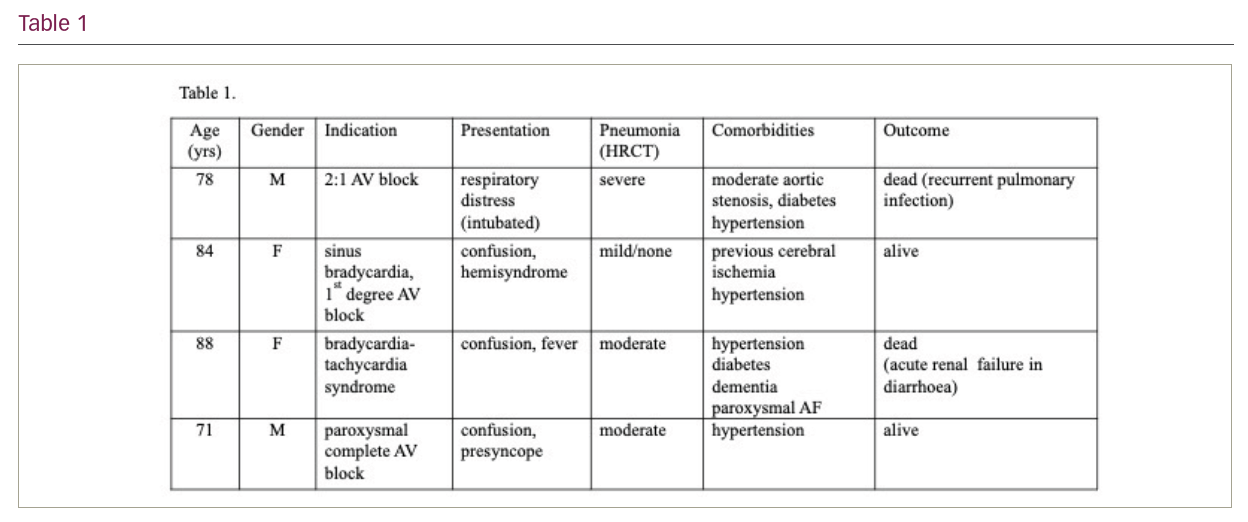
Results: In total, 1,168 patients were hospitalized (during the spring outbreak from 21/02/2020 to 31/05/2020, and during the autumn phase, from mid-October until the end of 2020), mean age 72 years, 42% were females. All had a positive molecular nasopharyngeal swab for SARS-CoV-2 at admission. All but 5 were admitted because of COVID-19-related pneumonia requiring oxygen supplementation. The COVID-19 treatment was standardized according to the best knowledge of the time (including hydroxychloroquine and lopinavir during the spring outbreak and plasma/remdesivir during the autumn). In-hospital mortality was 22.3%. Four patients received a transvenous pacemaker (one during the spring wave and three in the autumn phase). The clinical information is summarized in Table 1. Only one subject had an overt SARS CoV-2 pneumonia at presentation and had an underlying aortic disease. A second pacemaker was implanted because of conduction disease in a patient with only incidental positivity at swab without any clinical manifestation of COVID-19. Two patients had moderate pneumonia on HRCT, but one was implanted late after pneumonia resolution, during readmission because of bradycardia. No peri- and post-procedural complications were observed. Personnel was negative on serial swabs. Two patients died during the follow-up (five months and two weeks after implantation, respectively).
Conclusions: In this large cohort of COVID-19 patients with symptomatic pneumonia, the pacemaker implantation rate was not higher than the implant rate in the general population. The bradyarrhythmias might not be necessarily related to the SARS-CoV-2 infection. The procedure may be performed safely both for the patient and for the personnel. The outcome is related to the severity of COVID-19 disease and individual patient factors.