Background: Subcutaneous implantable cardiac defibrillators (S-ICDs) offer defibrillation protection therapy while avoiding lead-related complications associated with traditional ICDs. A major predictor of S-ICD eligibility is the T:R ratio. Despite current screening processes, T-wave oversensing remains the commonest cause of inappropriate shocks in S-ICD patients. The concept of varying S-ICD eligibility, owing to the dynamicity of electrocardiogram (ECG) signals, has been introduced before.
Purpose: There are practical limitations to acquiring longer durations of ECG signals for S-ICD screening. Machine learning methods are already in use for the classification of various cardiovascular diseases through ECG data analysis. This study explores the potential use of deep learning methods in S-ICD screening.
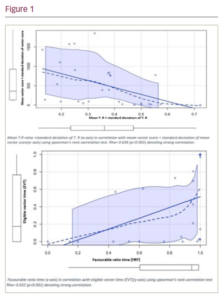
Methods: This was a retrospective correlation study. A deep-learning tool was used to provide descriptive analysis of the T:R ratios over 24-hour recordings of S-ICD vectors in adult ICD patients. Spearman’s rank correlation test was used to statistically compare the screening outcomes of the deep-learning tool with those of a ‘gold-standard’ S-ICD simulator for the same group of patients. Favourable ratio time (FVR) is a new concept introduced in this study representing the duration of time when the T:R ratio of a vector was deemed favourable (below the eligibility threshold) as a percentage of the whole recording. This was compared with the eligible vector time (EVT), which is the percentage of all the screening assessments with passing vector scores given by the S-ICD simulator.
Results: A total of 14 patients (mean age 63.7 ± 5.2 years, 71.4% male) were recruited, and 28 vectors were analysed. Mean T: R was 0.21 ± 0.11, standard deviation of T: R (as a measure of dynamicity) was 0.08 ± 0.04, and FVR was 79 ± 30%. Overall, there were statistically significant strong correlations between the outcomes of our deep-learning tool and the S-ICD simulator. Mean T:R ratio +standard deviation of T: R correlated strongly with mean vector score (measured via the S-ICD simulator) + standard deviation of mean vector score, Rho= 0.636 (p<0.001). FVR also strongly correlated with EVT (Rho= 0.652; p<0.001) (Figure 1).
Conclusion: Deep learning methods could provide a practical software solution to analyse data acquired for longer durations than current S-ICD screening practices. This could help select patients better suited for S-ICD therapy as well as guide vector selection in S-ICD eligible patients. Further work is needed before this could be translated into clinical practice. ❑