Introduction: Cardiac implantable electronic devices (CIEDs) therapy can be associated with complications. The European Society of Cardiology guidelines on pacing recommend routine follow-up of all newly implanted devices within 72 hours of implant. The European Heart Rhythm Association expert consensus and practical guide on CIEDs recommend that a chest X-ray should be performed within 24 hours after all CIED implants to rule out pneumothorax and to document lead positions.
Purpose: We aimed to report first on the rate of peri-procedural complications associated with CIED implants at our centre and identify any patient- and procedural-related factors that are associated with peri-procedural complications. We also report on the 3-month re-intervention rates at our centre.
Methods: Consecutive CIED implants performed between January and December 2019 were retrospectively analysed. Patients’ demographics, procedural reports, device checks, post-procedure chest X-rays and further intervention procedures were obtained from the hospital records. Data analyses were performed using RStudio 1.4.1106 running R 4.0.5. Categorical data are presented as n/N (%) and continuous data as mean ± SD. Fisher’s exact and chi-squared tests were used to analyse categorical variables’ contingency tables, while continuous non-parametric data were compared using Wilcoxon rank sum test.
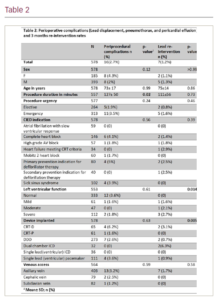
Results: A total of 578 patients were included in our analysis (Table 1). There were 16 (2.8%) peri-procedural complications, 7 (1.2%) pneumothoraxes, 6 (1%) pericardial effusions and 3 (0.5%) lead displacements. Axillary vein was the most common route of access (71%) followed by subclavian vein (15%) and cephalic vein (14%). All the pneumothorax cases occurred with axillary vein access; 2 out of the 3 lead displacements occurred after cephalic vein access and the other lead displacement happened after axillary access; 5 pericardial effusions followed axillary access while the remaining case followed subclavian vein access. Overall, 81.3%, 12.5% and 6.2% of the peri-procedural complications occurred after axillary, cephalic and subclavian vein access, respectively (p=0.59). The only parameter that correlated significantly with peri-procedural complications was the procedure time; the average procedure time in uncomplicated cases was 99 ± 43 minutes vs 127 ± 50 minutes in procedures associated with peri-procedural complications (p=0.02) (Table 2). The overall 3-month lead re-intervention rate was 1.2%: 4 lead revisions were required due to unsatisfactory parameters upon subsequent follow-up checks and 3 lead revisions were performed secondary to lead perforations. In 100% of the lead re-intervention cases, the primary CIED implant procedure was done via axillary vein access.
Conclusion: Axillary access for CIED implant was associated with higher rates of peri-procedural pneumothorax and subsequent lead re-intervention. The only statistically significant predictor of peri-procedural complications was the duration of the procedure. ❑