Introduction: In October 2020, Forth Valley Royal Hospital’s (FVRH) pacing service underwent a full repatriation from the Royal Infirmary of Edinburgh. Interventional radiology (IR) was established as the main inpatient pacing site (2 sessions a week) with IR staff education, training and developing standard operative procedures. Theatre capacity was increased from 1 to 2 sessions per week for elective pacing and the pacing services expanded to include complex devices, subcutaneous implantable cardioverter defibrillator and His bundle pacing. This report compares data from 1 year prior to the repatriation date (Year 1) and 1 year post (Year 2), in order to assess the quality of the new service provided and impact on the local population.
Methods: Data were collected on all patients undergoing an implant procedure within the hospital and entered into an Excel spreadsheet. This included: date of implant, type of device implanted, if procedure occurred in IR or theatre, if patient was inpatient (I/P) or outpatient (O/P), admission and discharge date, and length of stay. Complication and re-intervention rates were collected for Year 2.
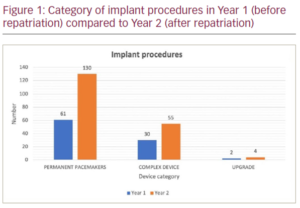
Results/Discussion: There were 96 patients who received an implant procedure at FVRH in Year 1 which increased to 194 patients in Year 2. From Year 1 to Year 2, the number of patients receiving implants increased in all device categories (permanent pacemakers, complex devices, generator replacements and upgrades) (Figure 1). Of the 65 O/Ps in Year 1, 71% were discharged the same day and 29% were discharged the day after. This increased in Year 2 with 75% of the 123 O/Ps being discharged the same day. I/P average length of stay decreased from 15.1 days in Year 1 to 4 days in Year 2 with most patients being discharged within 24 hours of implant. The waiting time for routine elective device implantation dropped from 3 months in Year 1 to 2 weeks in Year 2. Total complication rate from all implant procedures in Year 2 was low, at 3.6% with a re-intervention rate of 2.1% for permanent pacemakers and 4.7% for complex devices. This is less than the national 1-year re-intervention rate of 4.3% for permanent pacemakers and 6% for complex devices.1
Conclusion: The development of a local comprehensive cardiac devices service in NHS Forth Valley had a positive effect on providing a person-centred approach that improved patients’ journeys and reduced in-hospital bed stay and need for inter-hospital transfers. The service continues to be safe and efficient with low complication rates. In Year 3, the NHS Forth Valley cardiac devices service has successfully expanded further in order to provide regional devices implantation support to patients from NHS Fife and NHS Dumfries and Galloway, underpinning the need for collaborative work between different health boards in order to provide a successful and innovative health service for the wider population. ❑
- The National Institute for Cardiovascular Outcomes Research. National audit of cardiac rhythm management (NACRM), 2021 summary report. P.34. [internet]. 2021 [28/03/22]. Available at: https://www.nicor.org.uk/wp-content/uploads/2021/10/NACRM-Domain-Report_2021_FINAL.pdf







