Transcatheter aortic valve replacement (TAVR) has seen worldwide explosive growth. The current USA guidelines no longer use risk in choosing between TAVR and surgical aortic valve replacement, and the European guidelines have extended the recommendation for TAVR to lower risk groups.1,2 This rapid expansion has been driven by data from randomized trials showing TAVR as either non-inferior or superior to surgery.3–6 The ACURATE neo2™ system (Boston Scientific, Marlborough, MA, USA) was declared CE marked by the manufacturer for use in Europe in higher-risk patients. The current ACURATE IDE trial (NCT03735667) will test the ACURATE neo2 valve against commercially available valves in all risk categories in the USA.7 This manuscript will discuss the valve system, the current data and why this valve may be a good choice in all risk groups, but particularly in younger, lower-risk patients.
The ACURATE neo2 valve maintains key features of the ACURATE neo™ while introducing new innovations designed to alleviate some of the most common complications of TAVR (Figure 1). The valve is comprised of a self-expanding nitinol frame with porcine pericardium leaflets in a supra-annular position, which favours large effective orifice areas (EOAs) and low transvalvular gradients.8,9 The top-down, two-step deployment provides haemodynamic stability during the procedure, with annular co-axial alignment aided by the stabilization arches. The radial force exerted by the valve frame is relatively low, reducing the risk of annular rupture and mechanical injury during implantation.8 The ACURATE neo2 has an open-cell design, which facilitates coronary access, and the upper crown both anchors the valve to reduce the risk of embolization and captures the native valve leaflets to provide coronary clearance.8,10 The lower crown of the valve protrudes minimally into the left ventricular outflow tract to reduce the risk of conduction system interference.8,9
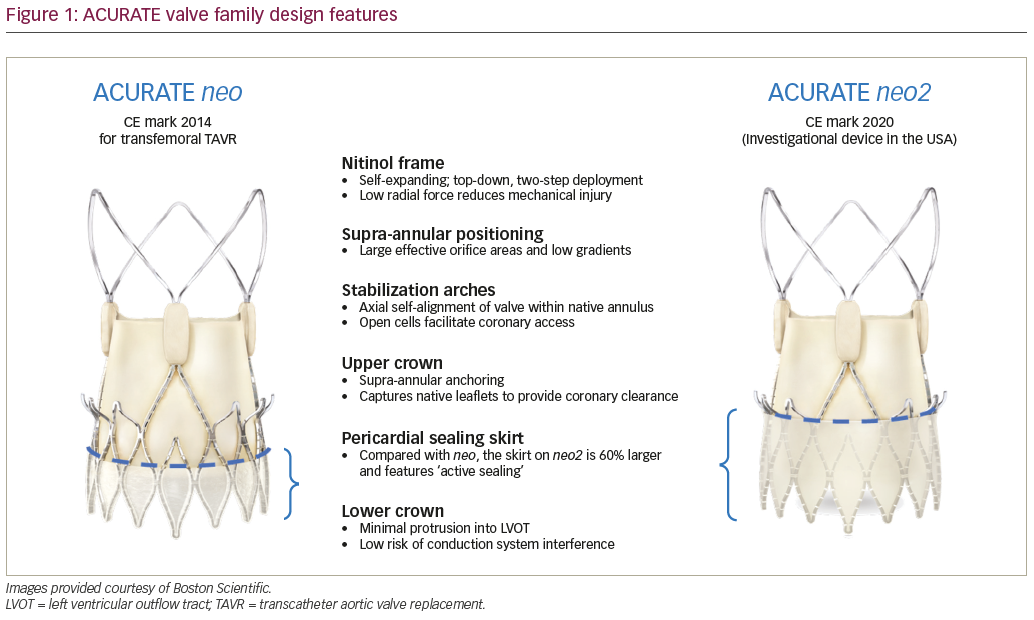
While both the neo and neo2 valves have a pericardial sealing skirt to reduce paravalvular leak (PVL), the skirt on the ACURATE neo2 is 60% larger, reaching to the waist of the stent. Additionally, the flexible ACURATE neo delivery catheter has been upgraded with a new atraumatic tip design, and when coupled with the low-profile expandable introducer, is able to accommodate a wide range of complex patient anatomies. The ACURATE neo2 also features a new radiopaque positioning marker to enhance visualization and accuracy. Valve sizing remains the same, with the largest valve treating up to a 27 mm annulus, and the addition of a larger valve planned. Valve crimping and loading remains essentially unchanged in the new system.
ACURATE clinical evidence
Initial safety and effectiveness of transfemoral TAVR with the ACURATE neo valve were demonstrated in the TF89 ‘CE-approval cohort’ (NCT03003650) and confirmed by the 1,000-patient SAVI-TF post-market registry (NCT02306226).11–13 SAVI-TF had a procedural success rate of 98.7%, with no instances of annular rupture, ventricular septal perforation or coronary obstruction requiring intervention, and no cases of endocarditis or valve thrombosis through 30 days. Patients exhibited favourable clinical outcomes – the 1-year mortality rate (8.0%) was comparable to that observed in large registries of Sapien 3 (Edwards Lifesciences, Irvine, CA, USA) (11.8% in SOURCE 3; NCT02698956), CoreValve (Medtronic, Minneapolis, MN, USA) (16.0% in ADVANCE ; NCT01074658), and Lotus (Boston Scientific, Marlborough, MA, USA) (11.7% in RESPOND; NCT02031302) – and the rate of permanent pacemaker implantation was low (8.3% at 30 days and 9.9% at 1 year).14–19 Patients in SAVI-TF exhibited low gradients and large EOAs (Table 1), as expected for a supra-annular valve, and a low rate of moderate or greater PVL at hospital discharge (4.1%) and at 1 year (3.6%).8,9,11-13,20–30
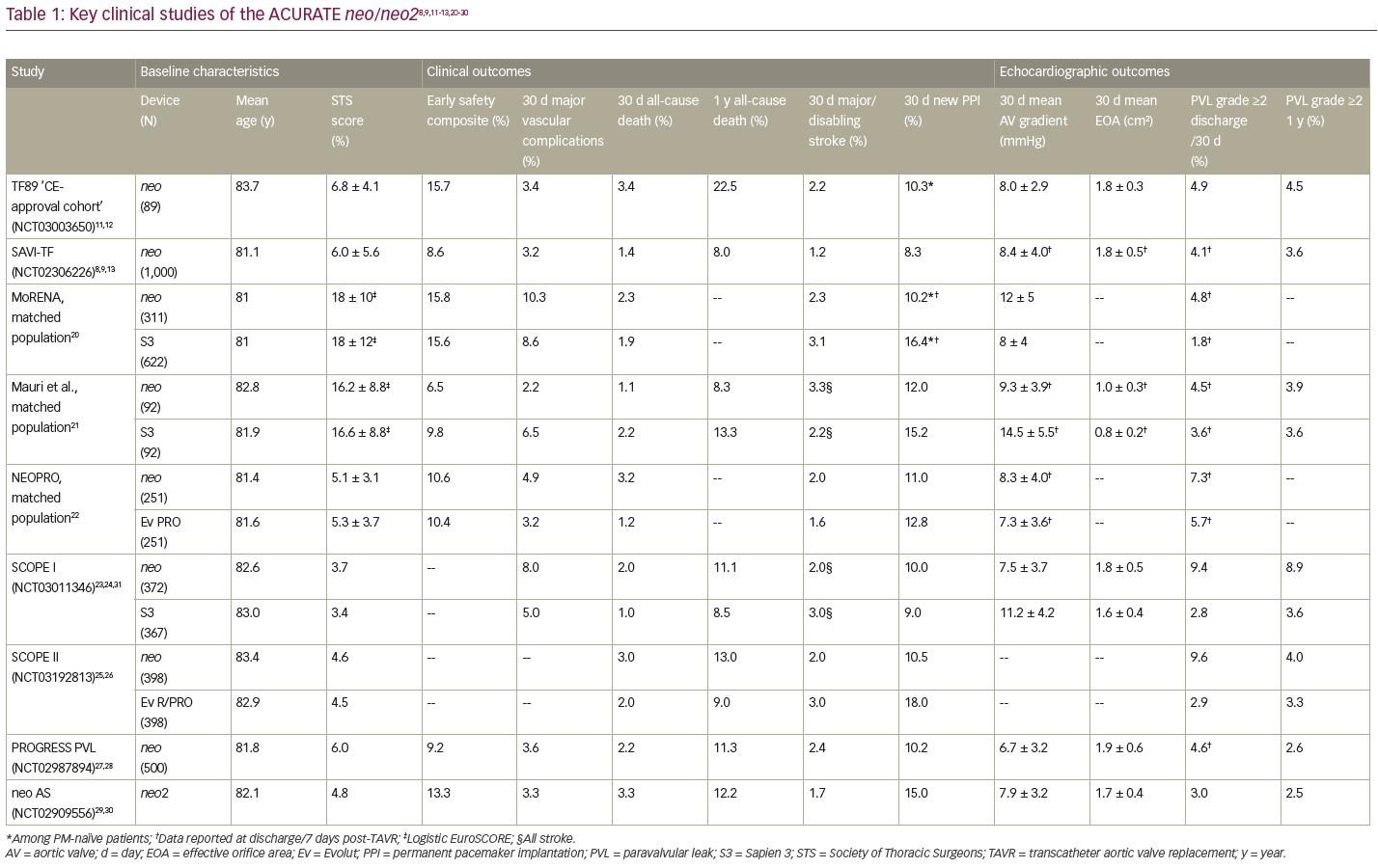
A number of independent head-to-head studies have compared the ACURATE neo to contemporary competitor devices (Table 1). While the neo was shown to perform well over a variety of procedural and clinical outcomes, these studies have produced conflicting data regarding PVL. In two separate propensity-matched comparisons between the ACURATE neo and the Sapien 3, the MoRENA registry and a study by Mauri et al., there was no difference between the devices in terms of procedural success or safety outcomes.20,21 In both of these studies, the ACURATE neo was less likely to result in patient–prosthesis mismatch, with significantly better transvalvular mean gradient and EOA compared with Sapien 3, and patients treated with the neo had a significantly lower rate of permanent pacemaker implantation. While the incidence of PVL grade ≥2 at discharge was significantly greater with the neo versus Sapien 3 in the MoRENA study (4.8% versus 1.8%, respectively; p=0.008), the difference between devices was not significant in the Mauri et al. study (4.5% versus 3.6%; p=0.208). The NEOPRO registry, which included a propensity-matched comparison of the ACURATE neo and Evolut PRO, likewise found no significant differences in procedural success or 30-day clinical safety outcomes between the matched pairs.22 In this study, patients treated with the ACURATE neo had significantly higher AV gradients, but the incidence of moderate or greater PVL was similar between the two groups (7.3% versus 5.7%; p=0.584).
More recently, the SCOPE I (NCT03011346) and SCOPE II (NCT03192813) studies evaluated outcomes in patients randomized to treatment with the ACURATE neo versus the Sapien 3 and the Evolut™ R/PRO (Medtronic, Minneapolis, MN, USA), respectively.23–26 In the SCOPE I study, the ACURATE neo did not meet non-inferiority criteria compared with the Sapien 3 on the 30-day composite primary endpoints of all-cause mortality, stroke, life-threatening/disabling bleeding, major vascular complications, coronary obstruction, acute kidney injury, rehospitalization, repeat intervention and valve dysfunction (23.7% versus 16.5%; p=0.42).24,31 Patients treated with the ACURATE neo were at greater risk for acute kidney injury (3% versus 1%; p=0.03) and moderate or greater PVL (9.4% versus 2.8%; p<0.001) at 30 days. A secondary analysis found that, for the individual components of death, stroke, rehospitalization, bleeding and vascular complications, outcomes were not significantly different between the two devices at either 30 days or 1 year.23,31 Conduction disturbances were low for both valves, with new permanent pacemaker implantation rates ≤10%, and patients in SCOPE I treated with the ACURATE neo had more favourable gradients and valve areas compared with those treated with the Sapien 3, which led to statistically lower rates of prosthesis–patient mismatch for the neo (p<0.001).31
In the SCOPE II study, the primary endpoint was all-cause death or stroke at 1 year, evaluated in both the intent-to-treat (ITT) and per-protocol populations to establish non-inferiority.26 While the ACURATE neo did not meet non-inferiority criteria versus Evolut R/PRO in the ITT analysis (15.8% versus 13.9%; p=0.05), thus missing the primary endpoint, it did meet non-inferiority criteria in the per-protocol analysis (15.3% versus 14.3%; p=0.03).25 Patients treated with the ACURATE neo were shown to have a greater risk for cardiac death at 30 days (2.8% versus 0.8%; p=0.03) and 1 year (8.4% versus 3.9%; p=0.01), as well as more frequent incidence of moderate or severe PVL at 30 days (9.6% versus 2.9%; p<0.001).25 However, the ACURATE neo did demonstrate a statistically significant advantage over the Evolut on the secondary endpoint of permanent pacemaker implantation at 30 days (10.5% versus 18.0%; p=0.003) and 1 year (11.0% versus 18.3%; p=0.004).
The primary contributing factor to the ACURATE neo not achieving non-inferiority in the SCOPE studies was a greater incidence of moderate or greater PVL. Studies performed in a large single-centre cohort have shown that it is possible to reduce PVL rates for the ACURATE neo through careful attention to patient selection, including thoughtful sizing and consideration of aortic valve calcification.32,33 In this cohort, the rate of PVL grade ≥2 at discharge was 3.7%.33 Additionally, there appears to be a learning curve in the implantation technique. The PROGRESS PVL study (NCT02987894), which evaluated core laboratory-adjudicated echocardiographic data over time, included a large proportion of investigators with prior clinical experience with the ACURATE neo.28 The rate of procedural complications was low and the valve was correctly positioned in 98.6% of patients.27 As in prior neo studies, patients exhibited low transvalvular gradients and large EOAs overall. A paired analysis in a subset of patients (n=209) demonstrated improved PVL over the course of the study, with a rate of moderate or greater PVL of 4.3% at discharge and 2.9% at 1 year.27
In addition to patient selection and procedural considerations, the features incorporated into the next iteration of the ACURATE valve, neo2, are intended to reduce PVL in comparison to the prior generation ACURATE neo system. The addition of radiopaque markers to neo2 helps to facilitate highly accurate valve positioning, and the enhanced and extended pericardial sealing skirt features a supra-annular flap that actively seals during each cardiac cycle. Clinical and echocardiographic outcomes in patients treated with the ACURATE neo2 valve were investigated in the ACURATE neo AS study (NCT02909556).29,30
Patients in the ACURATE neo AS study achieved a high rate of procedural success (97.5%).29,30 There were no reinterventions for valve-related dysfunction and a low rate of major vascular complications (3.3%). Safety outcomes were consistent with results from prior studies of the ACURATE neo and comparable to those observed in other TAVI studies in similar patient populations. The VARC-2 composite safety endpoint rate at 30 days was 13.3%, which was lower than prior studies of the ACURATE neo (15.8% in the MORENA study; 16.4% overall in the NEOPRO study). Core laboratory-adjudicated data demonstrated significant early haemodynamic improvement, maintained through 12-month follow-up.29,30 The overall rate of moderate PVL at 30 days was 3.0%, comparable to the rate observed with the Sapien 3 (3.6% by Mauri et al.; 2.8% in SCOPE I) and the Evolut R/PRO (3.0% in SCOPE II), and an improvement over ACURATE neo.21,25,31 Overall, the ACURATE neo AS study demonstrated the safety and performance of the ACURATE neo2 valve in patients with severe aortic valve stenosis.
Recently, promising data on real-world outcomes with the ACURATE neo2 was presented from two investigator-led European registries. The ITAL-neo Registry, which evaluated 95 patients treated with the ACURATE neo2, reported a low rate of in-hospital stroke (1.1%) and excellent valve haemodynamics (8.2 mmHg pre-discharge mean gradient).34 In this study, 3.1% of patients exhibited moderate or greater PVL at discharge, 56.9% had mild PVL and 40% had no detectable PVL. The observed PVL rates for patients with data available at 30 days (34/95) were further reduced: 0% moderate or greater PVL, 53% mild PVL and 47% no PVL. In the Early neo2 Registry (NCT04810195), which collected data from 554 patients treated with the ACURATE neo2, patients had a low in-hospital stroke rate (2.1%), an in-hospital new PPI rate of 6%, excellent valve haemodynamics (post-procedural mean gradient of 9 mmHg) and a low 30-day mortality rate (1.3%).35,36 According to site-reported, post-procedure echocardiographic data, 1.3% of patients exhibited moderate/severe PVL, 33.3% had mild PVL and 65.4% had no/trace PVL.34 This study also included a retrospective analysis of core-lab adjudicated echocardiographic data from patients treated with the neo (n=108) versus the neo2 (n=120).36 The ACURATE neo2 was associated with a 5.5% absolute risk reduction of aortic regurgitation fraction (p<0.001) and the rate of moderate or severe aortic regurgitation was significantly lower with the neo2 compared with the neo (1.7% versus 13.9%; p<0.001).36
Summary
The ACURATE neo2 valve builds on the strengths of the well-tested ACURATE neo platform. The flexible delivery catheter allows for trackability through tortuous anatomy; radiopaque markers help to refine positioning; and a simple two-step, top-down deployment method allows for stable and predictable release, making it a reliable and relatively easy-to-use device. This ease of use, evidenced in the high rate of procedural success and low peri-procedural complication rates observed in clinical studies, will be an advantage as TAVR procedures become more commonplace and are performed in a wider patient population.
Extension of TAVR to younger, more active patients will require consideration for features related to long-term valve performance. As a supra-annular valve, the ACURATE neo offers a high degree of haemodynamic performance, with larger EOA and lower gradients compared with valves with an intra-annular leaflet position. The stent’s open-frame design allows for preservation of coronary access, which is particularly important for patients who may have follow-up procedures. As with all TAVR systems, the potential for re-intervention and valve-in-valve procedures should be considered; while TAVR-in-TAVR has been successfully performed with ACURATE, there is certainly a need for additional experience. The enhancements to the anti-leak skirt for ACURATE neo2 may further decrease PVL, which will be investigated in prospective clinical studies, including the currently enrolling ACURATE IDE Study comparing the ACURATE neo2 to either the Sapien 3 or the Evolut R/PRO.7 Continued commitment to head-to-head studies of TAVR devices will be critical in evaluating longer-term outcomes in a widening, diversified patient population.