Introduction: New safe sedation guidelines were introduced by the Academy of Royal Colleges in 2013. We subsequently designed and introduced a new protocol to guide the use of conscious sedation in our electrophysiology labs in 2014. The protocol included pre-procedural risk assessment, routine capnography monitoring, protocolised prescribing of midazolam and fentanyl, dose-up titration guidance and the non-routine use of flumazenil.
Methods: All EP procedures between 2014 to 2018 were reviewed. 9,210 case summary entries reviewed on local EP database to identify cases with any sedation related events. These were classified as over-sedation or respiratory arrest, procedure intolerance or flumazenil use. All Medcon reports searched for term “flumazenil” in medication field and summary text.
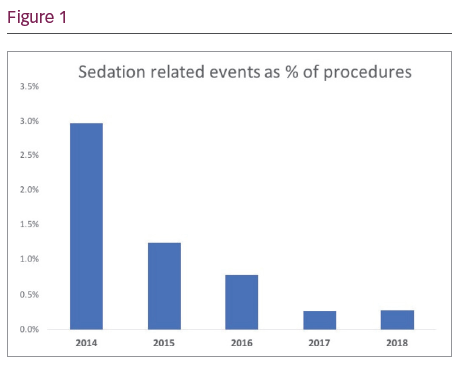
Results: Flumazenil use has significantly reduced from 40 cases in 2014 to zero in 2018. The number of over sedation or respiratory arrests has reduced from two in 2014 and two in 2015 to zero over the past 3 years. No increase has been seen in the number of procedures terminated due to agitation or uncontrolled pain using the new sedation protocol. Overall there has been a significant decrease in sedation related events seen from 2014 to 2018 (see Figure 1).
Conclusions: The introduction of a new safe sedation protocol to guide our EP lab physicians and nursing staff has significantly reduced the number of sedation related events recorded over the last 5 years without any increase seen in the number of procedures terminated early due to patient discomfort.