Coronary artery disease is the leading cause of morbidity and mortality globally.1 Dual antiplatelet therapy (DAPT), in the form of aspirin and a P2Y12 inhibitor, is the mainstay of pharmacotherapeutic treatment for acute coronary syndrome and prevention of stent thrombosis after percutaneous coronary intervention (PCI) for acute coronary syndrome or stable coronary disease. DAPT with ticlopidine plus aspirin was first shown to be superior to anticoagulation and aspirin for patients undergoing PCI in 1996.2 Since then, DAPT of clopidogrel plus aspirin has been shown to be superior to aspirin alone, with a relative risk reduction of 26.9% for major adverse cardiovascular events (MACE).3 DAPT has since become one of the most extensively investigated treatment strategies in cardiology, with over 35 randomised controlled trials and 225,000 patients, and is increasingly important given the volume of patients requiring DAPT. In 2015, European-based population estimates suggested that 1.4–2.2 million patients require DAPT per year.1
The duration of DAPT has evolved with the introduction of second- and now third-generation drug-eluting stents. First-generation drug-eluting stents raised the concern of very late stent thrombosis after 1 year,4 with evidence supporting prolonged duration of DAPT to prevent subsequent spontaneous myocardial infarction.5 This led to randomised controlled trials investigating prolonged duration of DAPT. The trade-off of reducing ischaemic sequelae is always balanced with the risk of bleeding, with evidence supporting a significant increase in bleeding events with 12 months of DAPT compared with >12 months of DAPT, with a minimal reduction in MACE (2.5% versus 1.6% major bleeding, respectively; p<0.001).6
Having established the safety of 1 month of DAPT after DCB angioplasty in stable coronary disease, we sought to review the evidence for shorter duration DAPT in terms of bleeding rates, clinical outcomes and safety profiles for both DCB and drug-eluting stents.
Identifying bleeding risk and risk stratification
Given the aging population and the increasingly complex co-morbidities that we are seeing, the risk of bleeding is greater. This has led to the introduction of bleeding risk stratification scoring systems both for clinical and research purposes. Both the DAPT and PARIS risk stratification scores were introduced based on prediction of events during index admission or shortly after.7,8 Neither of these stratification scores looked at the duration of DAPT in relation to bleeding risk. The most comprehensive scoring system to date is the PRECISE-DAPT scoring system, which is recommended by the European Society of Cardiology (ESC) guidelines as a class IIb A recommendation for use.1 The PRECISE-DAPT study showed that if patients considered at a high risk of bleeding were given a prolonged (>12 months) duration of DAPT, there was no ischaemic benefit but a significantly higher bleeding risk with a number needed to harm of 38.9
Guidelines
DAPT guidelines are different for acute coronary syndrome and PCI in stable coronary disease. Regardless of bleeding risk, DAPT is recommended for 12 months for all patients after acute coronary syndrome.1 However, for patients undergoing PCI for stable coronary disease, the evidence is less cohesive. This becomes relevant for two reasons:
- as a stable group, there is time to plan the intervention strategy, assess bleeding risk and determine an appropriate approach to an individual patient; and
- there is less clear-cut evidence on the duration of DAPT for this cohort.
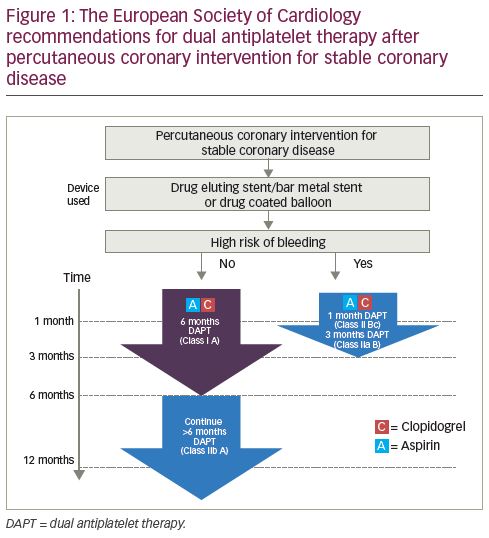
The current ESC guidelines for patients not at a high risk of bleeding with stable coronary disease treated with a drug-eluting stent/bare-metal stent or a drug-coated balloon (DCB), advise 6 months of DAPT (class I A recommendation) with a class IIb A recommendation to continue DAPT for a further 6 months (DAPT consisting of aspirin and clopidogrel) (Figure 1). For patients at a high risk of bleeding treated with drug-eluting stent/bare-metal stent or DCB, a 1-month duration of DAPT has a class IIb C recommendation, with 3 months of DAPT having a class IIa B recommendation. Despite these guidelines, routine clinical practice continues to give 12 months DAPT for patients receiving a drug-eluting stent for stable coronary disease unless there are significant bleeding sequelae.10
A systematic review and meta-analysis (17 studies, 46,864 patients) compared short-term (≤6 months but excluded those with ≤1 month) with standard duration of DAPT (12 months) and long duration DAPT (≥12 months) for drug-eluting stents. This included all patients with acute coronary syndrome and stable coronary disease. It showed a statistically significant increase in all-cause mortality and major bleeding in the long-duration DAPT group, and an increase in any bleeding in the standard-duration DAPT group compared with the short-term duration DAPT group, with no statistically significant difference in MACE.11 While this systematic review identifies a 6-month duration of DAPT as safe, it does not explore the evidence supporting a duration of DAPT of less than 6 months. This meta-analysis did not identify whether patients were at a higher risk of bleeding.
Drug-coated balloons – a practical alternative
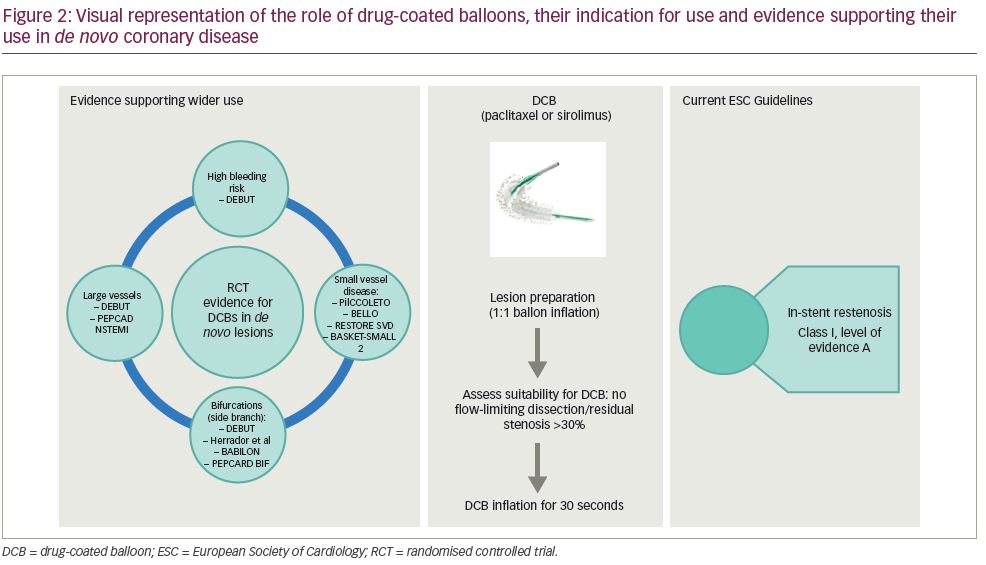
DCBs are an attractive proposition for cardiology interventionalists who subscribe to the ‘leave nothing behind’ philosophy.12 The use of DCBs is currently recommended by the ESC guidelines for in-stent restenosis. However, over the past 2 years, the evidence supporting the role of DCBs in wider circumstances has increased (Figure 2).13–27
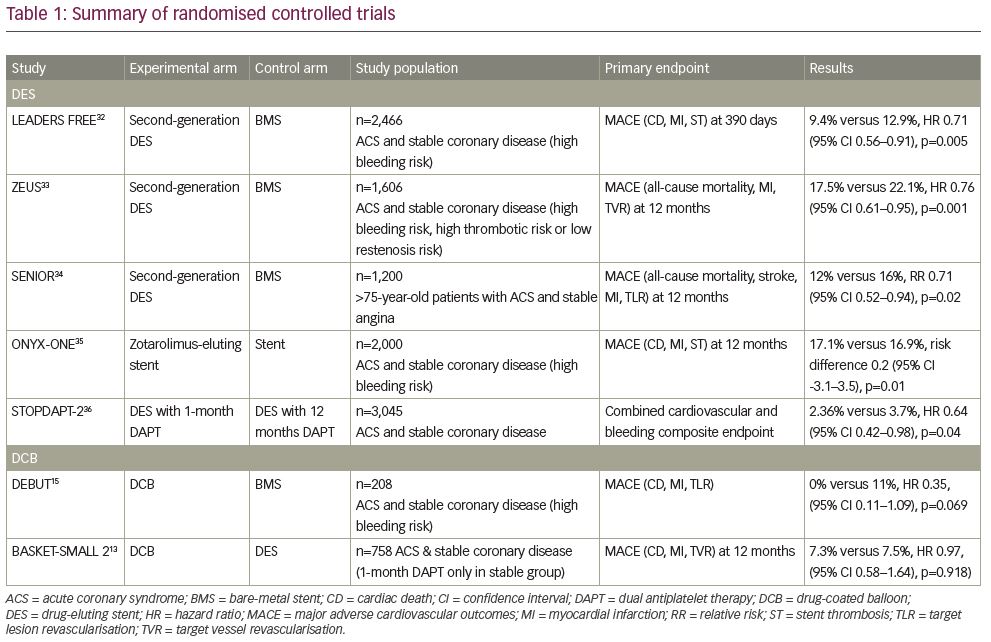
One significant benefit of a DCB strategy is the proposed shorter duration of DAPT required. Previous consensus groups have all recommended 1 month of DAPT for stable coronary disease.17,28,29 This recommendation was changed by the 2017 ESC Focused DAPT Update, which recommended a 6-month duration of DAPT after DCB angioplasty.1 In response to this, a recent retrospective analysis of real-world registry data in 303 patients treated with 1 month of DAPT after elective DCB angioplasty, reported no occurrence of MACE at 6 months, and found that 1 month of DAPT appears safe after DCB angioplasty for stable coronary disease.30 Further to this, recent work has confirmed long-term safety with 1 month of DAPT after elective DCB angioplasty,31 which is supported by the 36-month outcomes published in the BASKET-SMALL 2 trial.13 Table 1 provides a summary of all papers included in the review.13,15,32–36
Drug-eluting stents with 1 month of dual antiplatelet therapy – improved safety profile
There has been recent emphasis on identifying the safety of 1-month duration of DAPT for drug-eluting stents, given that studies suggest at least 15% of patients undergoing PCI are at a high risk of bleeding.37 Until recently, bare-metal stents were considered an appropriate strategy for patients at a high risk of bleeding, as 1 month of DAPT was deemed safe and adequate. This was despite all of the evidence showing superiority of drug-eluting stents compared with bare-metal stents, particularly in terms of target lesion revascularisation.38 In addition, there had been no safety or efficacy data supporting the use of drug-eluting stents for 1 month only. As such, significant advancements have been made in stent technology in improving the safety profile for a shorter duration of DAPT. This includes newer-generation drug-eluting stents, bioresorbable polymer and faster re-endothelisation combined with thinner struts, which all influence rates of stent thrombosis.39
The ZEUS, SENIOR and LEADERS FREE trials all changed perspectives on the use of bare-metal stents for patients with high bleeding risk, showing that the use of drug-eluting stents with 1 month of DAPT was superior in terms of safety and efficacy over the use of bare-metal stents with 1 month of DAPT.33,34,40 The LEADERS FREE trial randomised 2,466 patients, including those with acute coronary syndrome and stable coronary disease, to either second-generation drug-eluting stents or bare-metal stents with 1 month of DAPT. A primary safety endpoint of cardiac death, myocardial infarction or stent thrombosis showed drug-eluting stents to be superior to bare-metal stents (9.4% versus 12.9%, respectively; hazard ratio [HR] 0.71; 95% confidence interval [CI] 0.56–0.91; p=0.005). There was no statistically significance between bleeding events based on the Bleeding Academic Research Consortium (BARC) criteria for types 3–5 bleeding (7.2% for the drug-eluting stent versus 7.3% for the bare-metal stent; p=0.96).40
The ZEUS study compared second-generation drug-eluting stents with bare-metal stents in a more heterogeneous population – those at high bleeding risk, high thrombotic risk or low restenosis risk. A subgroup analysis of patients at a high bleeding risk (n=828) favoured drug-eluting stents over bare-metal stents with a primary composite outcome of death, myocardial infarction or target vessel revascularisation (HR 0.74; 95% CI 0.57–0.97).33
The SENIOR trial randomised 1,200 patients over the age of 75 to either drug-eluting stents or bare-metal stents, and gave 1 month of DAPT for stable angina and 6 months for acute coronary syndrome. Although these patients were not specifically at high risk of bleeding, their age does contribute to bleeding risk. The primary endpoint was a composite of all-cause mortality, myocardial infarction, stroke or ischaemia-driven target lesion revascularisation, and results favoured drug-eluting stents (12% versus 16% in the bare-metal stent group; relative risk 0.71; 95% CI 0.52–0.94; p=0.02). Bleeding complications occurred in 5% of both arms.34
These three studies have shown the superiority of drug-eluting stents over bare-metal stents in patients at high risk of bleeding who would benefit from a shorter duration of DAPT. Having identified that drug-eluting stents are superior to bare-metal stents in this situation, further studies have sought to evaluate the safety of 1-month duration of DAPT compared with a longer duration. The more recent ONYX ONE trial, comparing the Onyx™ zotarolimus-eluting stent (Medtronic, Dublin, Ireland) with the BioFreedom™ polymer-free drug-eluting stent (Biosensors International, Singapore) showed non-inferiority with 1 month of DAPT, although event rates were notably high with the primary composite safety endpoint (cardiac death, myocardial infarction, stent thrombosis) at 1 year (17.1% in the zotarolimus-eluting stent group versus 16.9% in the polymer-free drug-eluting stent group).41
The STOPDAPT-2 trial randomised 3,045 patients in Japan to either 1 or 12 months of DAPT after PCI (of which, 38% had acute coronary syndrome). A primary composite endpoint of cardiovascular and bleeding events (cardiac death, myocardial infarction, definite stent thrombosis, ischaemic or haemorrhagic stroke, or Thrombolysis in Myocardial Infarction risk score major or minor bleeding) showed superiority of 1-month DAPT (2.4% versus 3.7% in the 12-month DAPT group; HR 0.64; 95% CI 0.42–0.98; p=0.04).36 Of note, the majority of patients were at low to intermediate risk of bleeding.
Drug-coated balloons and 1 month of dual antiplatelet therapy
Two prospective studies have been conducted reporting cardiovascular outcomes and bleeding events using DCB in one arm and either drug-eluting or bare-metal stents in the other arm. The first study, the DEBUT trial, was a randomised controlled trial comparing bare-metal stents with DCBs in patients at high risk of bleeding. This included patients with stable coronary disease or acute coronary syndrome in the form of non-ST segment elevation myocardial infarction/unstable angina but excluded ST segment elevation myocardial infarction. Three lesions (two patients) in the DCB group required bailout stenting. The occurrence of a primary outcome of MACE in stable angina was 0% in the DCB group versus 11% in the bare-metal stent group (HR 0.35; 95% CI 0.11–1.09; p=0.069). DEBUT also reported a 13% bleeding rate at 9 months in the DCB group, with 11% in bare-metal stent group (p=0.59). This was in a high-risk bleeding cohort with 58% (DCB cohort) on an oral anticoagulant and 29% (DCB cohort) anaemic with additional risk factors for bleeding, including old age (>80 years old), stage ≥3 chronic kidney disease, thrombocytopenia, frailty, synthetic liver dysfunction and previous intracranial haemorrhage or cerebrovascular accident.15
The second study was the BASKET-SMALL 2 trial.13 This was a randomised controlled trial comparing drug-eluting stents with DCBs for small vessel disease in 758 patients with acute coronary syndrome and stable coronary disease. The primary objective was non-inferiority of DCB. The patients who received a DCB for stable coronary disease were given 1 month of DAPT and those who had an acute coronary syndrome were given 12 months. The majority of patients included were those with stable coronary disease (70% in the DCB group and 73% in the drug-eluting stent group). Risk of bleeding criteria were not specified in the patient cohort. MACE at 12 months were 7.3% in the DCB arm versus 7.5% in the drug-eluting stent arm (HR 0.97; 95% CI 0.58–1.64; p=0.9180). Major bleeding rates were low, at 1.1% in the DCB group versus 2.4% in the drug-eluting stent group (p=0.46). Lower rates occurred in the DCB cohort, but this was not of statistical significance.13
A retrospective database analysis of all patients receiving 1 month of DAPT, showing no occurrence of MACE at 6 months, further strengthens the safety argument for the use of DCBs in those at high risk of bleeding.30 While the current ESC guidelines recommend DCBs only for in-stent restenosis,42 there is randomised controlled trial evidence supporting their use in small vessels (<3 mm).13 With some observational data supporting the use of DCBs in large vessels, including left main stem lesions,23,31,43,44 and upcoming randomised controlled trials being considered to further investigate the use of DCBs in large vessels, their role in patients with high bleeding risk is thought to increase.
Acute coronary syndromes and duration of dual antiplatelet therapy
The current guidelines still recommend 12 months’ duration of DAPT for all patients with acute coronary syndrome, regardless of treatment strategy.1 Although the purpose of this review is to focus on stable coronary disease, it is worth briefly mentioning the evidence for DCBs and drug-eluting stents for acute coronary syndrome with 1 month of DAPT. Within the drug-eluting stent randomised controlled trials, patients with acute coronary syndrome made up a significant proportion of the numbers: 41% in LEADERS FREE,32 63% in ZEUS,33 46% in SENIOR,34 52% in ONYX ONE,35 and 38% in STOPDAPT-2.36 In comparison, the only data for 1-month DAPT in DCB in acute coronary syndrome are in DEBUT, where patients with acute coronary syndrome account for 46% of patients.15 BASKET-SMALL 2 gave 12 months of DAPT to all patients with acute coronary syndrome.13 Therefore, although the clinical outcomes in DEBUT are excellent for DCBs, there is currently a smaller body of evidence supporting the use of 1-month DAPT in patients with acute coronary syndrome with DCB.
Discussion
When comparing the DEBUT data (DCB)15 with the LEADERS FREE trial (drug-eluting stent versus bare-metal stents in high-risk bleeding patients),40 the bleeding rates reported in DEBUT are not as high as those reported in the LEADERS FREE trial, where bleeding events (BARC 1–5) were 18.1% versus 19.1% (drug-eluting stent versus bare-metal stent, respectively), compared with 13% versus 11% in DEBUT (DCB versus bare-metal stent, respectively). Although the DEBUT numbers are smaller, both studies evaluated high risk of bleeding. In comparison, the BASKET-SMALL 2 trial reported lower bleeding events, at 4% versus 9% (DCB versus drug-eluting stent, respectively), but this patient group was not identified as being at a higher risk of bleeding, which may explain the lower bleeding rates.13
Of particular interest, however, is the fact that although the bleeding rates were slightly lower in DEBUT compared with LEADERS FREE, the MACE rates were significantly lower in the DEBUT trial (1% for DCB) than both the LEADERS FREE trial (9.4%) and the ONYX ONE trial (17.1% for the zotorolimus-eluting stent).35 Of course, these MACE rates cannot be directly compared; however, it certainly adds strength to the concept that DCB is a very appealing strategy for patients at high risk of bleeding. This is backed up by our registry data with 0% MACE rates at 6 months in patients who received 1 month of DAPT.30
Where the LEADERS FREE, SENIOR and ONYX ONE trials all report high MACE occurrence in the drug-eluting stent arm (9.4%, 12% and 17.1%, respectively), the results of the Japanese STOPDAPT-2 were significantly lower, with MACE rates at 2.36%. One hypothesis for this could be the use of intracoronary imaging to optimise stent sizing in almost all patients, which is not standard western practice.
With the exception of the STOPDAPT-2 trial, DCB studies show a significantly lower MACE rate when compared with drug-eluting stents or bare-metal stents. This adds weight to the argument that DCB is an attractive proposition for patients who are at a higher risk of bleeding, particularly in the stable angina cohort where bleeding risk can be assessed pre-procedure and angioplasty strategy planned accordingly.
Limitations
While all of the included drug-eluting stent studies have been conducted with large numbers, the sample sizes in the DEBUT and BASKET-SMALL 2 trials were smaller, although both studies were adequately powered to answer their primary outcome. As the population in all of the included studies varied from those at high risk of bleeding to a heterogeneous cohort, no definitive subgroup meta-analysis can be conducted that would add any weight to the available data.
Conclusion
In conclusion, we are increasingly faced with a more complex patient cohort with higher risk of bleeding associated with DAPT. Although it is clear that 6 months’ duration of DAPT can be given with adequate effects on MACE with drug-eluting stents, the MACE rates remain high with only 1 month of DAPT in the drug-eluting stent randomised controlled trials. In comparison, 1 month of DAPT with DCB in the DEBUT study15 and in a large real-world registry analysis31 shows significantly lower MACE rates than the contemporaneous drug-eluting stent studies. This strengthens the viewpoint that DCB is a very attractive proposition for all patients with stable coronary disease identified as being at a high risk of bleeding.