Cryptogenic stroke (CS) is a brain infarction that cannot be clearly attributed to cardioembolism, large-vessel atherosclerosis or small artery disease, despite extensive clinical evaluation.1 It represents approximately one-third of all ischaemic strokes worldwide.2 Stroke represents a major public health crisis, as it is the fifth most common cause of death in the USA and a significant cause of preventable long-term disability.1 In the USA, the yearly cost of strokes is approximately US$34 billion, which encompasses healthcare costs, missed workdays and medications.1
Patent foramen ovale (PFO), being the most common congenital abnormality, is present in about 25% of the general population.1,3,4 Retrospective studies have found that 40–50% of patients with a history of CS have a concomitant PFO.3–5 A PFO occurs when the embryological structure of the foramen ovale (FO) fails to close in the neonatal period. Changes in pressure within the right atrium (RA) lead to instances of PFO, increasing the risk for passage of a paradoxical thrombus into the systemic circulation.3 However, the relationship between PFO and CS is contested. Management of PFO with respect to reducing stroke is controversial. Here, we will review the history of PFO closure and the clinical trials that have evaluated the efficacy of PFO device closure.
Patent foramen ovale anatomy, embryology and definition
Septation of the atrial chambers occurs in the early embryonic period. The foramen primum is the initial large connection between the RA and the left atrium (LA). The septum primum (SP) forms towards the endocardial cushions, narrowing the FP.6 The foramen secundum develops simultaneously in the SP while maintaining right-to-left flow and leading to the FP eventually disappearing. The septum secundum partially overlaps with the SP, so the residual portion of the SP forms a FO. The SP and septum secundum fuse at birth due to higher LA pressure and decreased pulmonary vascular resistance. Failure to fuse leads to PFO.6
During foetal circulation, the umbilical vein carries oxygenated blood to the RA, which enters the LA through the FO and then into the aorta. This right-to-left shunt ensures the delivery of oxygenated blood in the foetus.6 Deoxygenated blood enters the RA, but bypasses the pulmonary system to be oxygenated due to high vascular resistance and eventually passes through the ductus arteriosus. Pulmonary vascular resistance decreases physiologically at birth, ultimately leading to higher LA pressure and favouring the closure of the FO.6
Patent foramen ovale diagnosis and associations
Different imaging modalities, such as transthoracic echocardiography (TTE), transoesophageal echocardiography, intracardiac echocardiography, transcranial Doppler (TCD), multi-detector computed tomography and cardiac magnetic resonance, are used to detect a PFO.7,8 TTE is often performed as a screening test due to its relative ease. It is performed at rest with the Valsalva manoeuvre to augment the shunt.9 Agitated saline is administered intravenously while monitoring for bubbles in the LA to assess the shunt volume and ensure high sensitivity in
detecting PFOs.8 Swan et al. demonstrated preferential shunting of blood flow from the inferior vena cava through the PFO.10 The timing of LA bubbles distinguishes intracardiac from transpulmonary shunts; LA bubbles within three cardiac cycles indicate intracardiac shunting.8 TTE can also further distinguish PFO from a true atrial septal defect (ASD) and identify an atrial septal aneurysm.1
Other modalities, such as TEE and TCD, can help identify a PFO. TEE can further characterize the PFO, detailing the tunnel length, anatomic structures and margins.1,9 The transcranial Doppler is unable to distinguish between cardiac versus extracardiac shunting.3 Multi-detector computed tomography and cardiac magnetic resonance can detect a PFO, but are less sensitive than transoesophageal echocardiography.7,8 Intracardiac echocardiography is a sensitive and specific modality, but requires right heart catheterization. It is the most accurate method for confirming the presence of a PFO.
Deep venous thrombosis (DVT) is also associated with PFO and may contribute to paradoxical embolism leading to CS.11 Retrospective data show an incidence as high as 20% of contaminant DVT in patients with CS and PFO.11 Work-up for CS should include imaging to evaluate for DVT.
Selecting patent foramen ovale closure
Defining which patients benefit from PFO closure has been a moving target. The Risk of Paradoxical Embolism (RoPE) score is often used to determine the likelihood of CS secondary to PFO.12 The score includes history of hypertension, diabetes, stroke or transient ischaemic attack, and smoking status, and cortical infarct on imaging and age. A higher RoPE score suggests a causal association between CS and PFO.12 Recently, the PFO-Associated Stroke Causal Likelihood Classification System, which incorporates the RoPE score with anatomic and clinical features of the PFO, has been proposed.13
The history of patent foramen ovale closure and cryptogenic stroke: The Gartner Hype Cycle
The Gartner Hype Cycle, first coined by the Gartner firm, is a graph representing the evolution of a novel trend or innovation.14 Traditionally, this model is used in the technology sector; however, it can be applied to many other fields – in this case, the history of PFO closure as it relates to CS (Figure 1).15–21
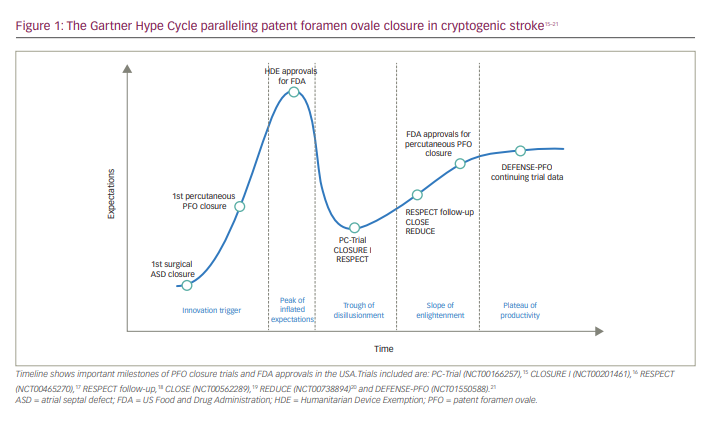
Technology trigger or ‘the trigger’
The surgical closure of ASD paved the way for percutaneous PFO closure. Paul Santy performed the first successful ASD closure in 1949 using RA appendage inversion.22 The advent of the cardiopulmonary bypass hastened the creation of the first percutaneous PFO closure device (King–Mills Cardiac Umbrella) in 1972 and its first human implantation in 1975.23 Since then, numerous closure devices have been created. Currently, two devices have been approved by the US Food and Drug Administration (FDA): the Gore® Cardioform Septal Occluder (WL Gore & Associates, Newark, DE, USA) and the Amplatzer™ PFO Occluder (Abbott Cardiovascular, Plymouth, MN, USA). Outside of the USA, there are several additional options that are currently awaiting FDA approval, including the Flex II PFO Occluder (Occlutech, Helsingborg, Sweden).
Peak of inflated expectations
The early 2000s brought new advances and approvals to PFO closure technology. The FDA granted Humanitarian Device Exemption status to CardioSEAL® STARFlex™ (NMT Medical, Boston, MA, USA) and Amplatzer™ PFO Occluder in 2000 and 2002, respectively.24,25 This exemption permitted their use for recurrent CS from a PFO after failed conventional medical therapy. However, by 2006, the number of devices being implanted exceeded the mandated annual limit of 4,000 patients, and the exemption was withdrawn.26
The CLOSURE I (Evaluation of the STARFlex® Septal Closure System in patients with a stroke or TIA due to the possible passage of a clot of unknown origin through a patent foramen cvale [PFO]; ClinicalTrials.gov identifier: NCT00201461) and RESPECT (Randomized evaluation of recurrent stroke comparing PFO closure to established current standard of care treatment [RESPECT]; ClinicalTrials.gov identifier: NCT00465270) trials started enrolling patients in 2003, while the REDUCE trial (GORE® Septal Occluder device for patent foramen ovale [PFO] closure in stroke patients [REDUCE]; ClinicalTrials.gov identifier: NCT00738894) started enrolling patients in 2008.16,17,20 There was almost palpable excitement surrounding potential new treatments for CS. During enrolment, many physicians and patients were reluctant to assign or be assigned to the medical treatment arm, perceiving device closure to be superior.1 This perception was perhaps more attributable to device implanters and trial design. In CLOSURE I, the original protocol relied on recruiting 1,600 patients to reach statistical significance, assuming an incidence of 3% of the primary endpoint in the device-therapy arm.16 Despite the enthusiasm, patient recruitment was slow. The trial was revised to an expected event rate in the device arm to 2%, and the sample size was reduced to 800 (909 patients were included in the final analysis).16 The reduced expected event rate with device closure followed a re-evaluation of what turned out to be inflated estimates of stroke recurrence in patients with PFO. This overestimation, based on Mas et al. from 2017, found that patients with a PFO and atrial septal aneurysm had an increased risk of recurrent stroke of 4.4%.19 This trial only involved 51 such patients, perhaps limiting its use as an accurate predictor.
Trough of disillusionment
Each peak, according to the Hype Gartner Cycle model, precedes a trough, and the Mas et al. trial data prompted the trough. The CLOSURE I trial randomized patients to medical therapy versus closure with the CardioSEAL® STARFlex™ Septal Occluder together with medical therapy.16 Results were published in 2012 and concluded that PFO closure with this device did not produce significant benefit compared with medical therapy alone.16 The RESPECT trial randomized patients to medical therapy versus PFO closure with the Amplatzer™ PFO Occluder.17 Initial results were published in 2013, with a mean follow-up of 2.6 years, and did not show a significant difference in recurrent stroke in the intention-to-treat analysis.17 The PC-Trial (Randomized clinical trial comparing the efficacy of percutaneous closure of patent foramen ovale [PFO] with medical treatment in patients with cryptogenic embolism; ClinicalTrials.gov identifier: NCT00166257) was conducted throughout 29 sites in Europe, Canada, Brazil, and Australia and randomized patients to medical therapy versus closure with the Amplatzer PFO Occluder.15 Results were published in 2013 and, similarly to the findings of the CLOSURE I trial, did not show a statistically significant benefit in secondary prevention of stroke in the device-closure arm.15,16 These three ‘negative’ trials precipitated discouragement toward percutaneous CS treatment, thereby leading to fewer PFO closures.1
Slope of enlightenment
The REDUCE trial randomized patients to antiplatelet therapy alone or in addition to PFO closure with either the Gore® Helex® Septal Occluder (WL Gore & Associates, Newark, Delaware, USA) or the Gore® Cardioform Septal Occluder device.20 Results published in 2017 showed a statistically significant reduction in recurrent ischaemic stroke in the closure group compared with the antiplatelet-only group. This trial directly led to the FDA expanding the approved indications for the Gore® Cardioform Septal Occluder in March 2018.27
A further review of the PC, CLOSURE I and RESPECT trials yielded additional insight. Approximately 5.7% of patients in the device arm of the CLOSURE I trial developed atrial fibrillation (AF) post procedure, which may have diminished the efficacy of this device.16 Moreover, had the study involved 1,600 or more patients, as originally planned, and followed up patients for 5 years, rather than the 2 years analyzed, there might have been a larger separation between trial arms. The PC-Trial did show fewer recurrent strokes in the closure group compared with medical therapy alone, with a non-statistically significant trend towards benefit with device closure.15 This trend was likely diluted by a high rate of crossover of patients from the medical treatment group to the device closure group.
The RESPECT trial found recurrent strokes in 9 patients in the closure group, 3 of whom developed strokes prior to closure device placement.17 Based on the prespecified intention-to-treat design, the RESPECT trial did not reach statistical significance for its primary endpoint; however, the as-treated analysis indicated the superiority of device closure compared with medical therapy alone. This ad hoc analysis prompted the FDA to request additional long-term follow-up results from this patient cohort. These extended results, with a mean follow-up of 5.9 years, were presented in October 2015 and published in September 2017.18,28 Prolonged follow-up yielded a statistically significant reduction in recurrent stroke in the closure arm in the intention-to-treat analysis as well.29 The FDA then approved the Amplatzer PFO Occluder device in October 2016.1,28
These two FDA-approved devices were made acceptable for treating PFO closure in the USA in patients aged 18–60 years who had a CS presumed from paradoxical embolism as determined by a neurologist and a cardiologist after an evaluation has excluded known causes of ischaemic stroke.30,31
Plateau of productivity and future directions
As growth continues for the PFO closure device field, new devices will emerge. There are several potential areas for new PFO closure devices tailored to different anatomical subsets. There is an increasing need for smaller LA ‘footprints’ to minimize difficulty with future transcatheter interventions involving the LA and for devices more suited for anatomical PFO subsets, such as those with long tunnels or highly mobile redundant septums, or even bioresorbable devices.1 The superiority of device closure over medical therapy continues to become more evident with time. This is particularly noticeable with the results of the RESPECT extended follow-up, which showed a persistently lower rate of recurrent stroke up to 10 years post-PFO closure compared with medical therapy.29 Participants in the RESPECT trial continue to be followed up, and it is believed that this protective effect will be seen in years to come. Discussions surrounding PFO closure devices should migrate away from whether they should be performed and towards which patient populations are most appropriate.
Lastly, there is a need for a national standardized registry to facilitate long-term follow-up monitoring, such as those that exist for other percutaneous cardiac procedures, including transcatheter aortic valve replacement, percutaneous mitral valve repair and LA appendage occlusion. This national standardized registry has yet to be established for PFO closure but would advance the knowledge and improve management of this patient population.
Treatment
The percutaneous treatment for PFO, which has been highly controversial, has now been validated in numerous well-controlled clinical trials. The focus should be geared towards selecting appropriate patients for PFO closure. The selection process requires a multi-disciplinary process including both cardiologists and neurologists, often with additional input from haematologists and other specialists.3 Patients younger than 60 years with a history of CS and a PFO should be evaluated for potential closure. Further workup should rule out other potential causes of stroke, such as arterial dissection, hypercoagulable states, uncontrolled hypertension or diabetes, alcohol or drug use, and autoimmune diseases.3
Cardiac arrhythmia, specifically AF, should be investigated with long-term monitoring. The monitoring period is variable and based on several factors, including age, electrocardiogram and echocardiography findings, and historical predisposition to AF; however, 30-day monitoring is generally the starting point.3
Valvular disease, cardiomyopathy and cardiac tumours should also be investigated, and limited life expectancy should be considered. Patients without these conditions should be considered for percutaneous closure of PFO. However, for patients with these conditions, medical therapy might be preferable to PFO closure.3 Medical therapy would include antiplatelet and/or anticoagulant medications. Randomized controlled trials have not shown that anticoagulant therapy is superior to antiplatelet therapy in patients contraindicated to PFO closure. However, the ultimate treatment choice depends on the patient and their comorbidities, which may guide a physician to select antiplatelets or anticoagulants.
Discussion
The data described above have demonstrated the strong relationship between PFO and CS, with PFO closure preventing strokes in some patients. The robust data supporting PFO as a cause of stroke bring to light the question of whether CS should be redefined as PFO-associated paradoxical stroke. The role of PFO closure in CS is now clear, though many unanswered questions remain. Most major clinical trials, including RESPECT, REDUCE and CLOSURE I, have targeted only patients aged 18–60 years.16,17,20 Thus, there is limited evidence supporting closure in patients aged over 60 years. Realistically, however, CS disproportionately affects older patients. In a recent meta-analysis, the prevalence of CS was lower in patients under 50 years.32 The clinical management for patients over 60 years is heavily individualized. The DEFENSE-PFO trial (Device closure versus medical therapy for cryptogenic stroke patients with high-risk patent foramen ovale [DEFENSE-PFO]; ClinicalTrials.gov identifier: NCT01550588) included a broader patient population, aged 18–80 years, and supports the role of PFO closure in older patients.21
The best medical therapy for patients, while studied, is not definitive. Current strategies include anticoagulant or antiplatelet medications. To date, there are insufficient data comparing anticoagulants and antiplatelets. Given that thromboembolism is the speculated source of CS, anticoagulants were thought to possibly be superior; however, a recent meta-analysis from 12 databases (including 2,385 patients) compared anticoagulants with antiplatelets in patients with CS and PFO and found no statistically significant differences.33,34 Clinicians often cite the treatment estimates from this study that slightly favoured anticoagulation. A review of current practising patterns revealed that, of the patients with CS receiving medical therapy, two-thirds received antiplatelets, while the other one-third received anticoagulants.34
The guidelines surrounding PFO closure and CS within the American scientific bodies have been continuously evolving (Table 1).9,16,19 In 2011, at the ‘peak of inflated expectations’ based on observational data, the American Heart Association/American Stroke Association (AHA/ASA) guidelines gave PFO closure a Class IIb recommendation, stating that there were insufficient data to make a higher-level recommendation.35 As multiple ‘negative’ trials were published (PC-Trial, CLOSURE I, RESPECT), the AHA/ASA downgraded their recommendation to Class III, mirroring the ‘trough of disillusionment’.36 The 2021 AHA/ASA guidelines reversed the negativity surrounding PFO closure.37 In fact, multiple recommendations were made relating to the role of PFO closure, one of which was a Class I recommendation. However, this Class I recommendation focused on the role of interdisciplinary decision-making between cardiology, neurology and the patient. A deeper look at the guidelines related to direct PFO transcatheter closure reveals, at best, a Class IIa recommendation for a subset population with high-risk anatomic PFO features. These changes to the guidelines mirror the Gartner Hype Cycle, but are still trailing behind the recent data. Similarly, the same school of thought is seen with the American Academy of Neurology, whose 2020 update uses somewhat weak language surrounding PFO closure, with Level C recommendations.38

European literature often precedes North American guidelines. In 2018, the European Heart Journal published a position paper with statements strongly in favour of PFO closure.39 Patients aged 18–65 years with a confirmed CS, transient ischaemic attack or systemic embolism with a high probability of a causal role of their PFO should undergo percutaneous PFO closure.39 Although the North American guidelines have been updated at different stages, treatment gaps remain. From a clinician standpoint, these guideline gaps create clinical turbulence, insurance non-coverage, and patient confusion surrounding PFO closure. Hopefully, the next iteration of AHA/ASA guidelines will contain precise language to accurately reflect current clinical data.
Conclusion
This multi-part review collates PFO embryology, diagnosis, clinical trials and the current guidelines for PFO closure. The Gartner Hype Cycle is used as an analogy for the clinical trial history of PFO closure for stroke prevention. In addition, we reviewed current guidelines, including those from the AHA/ASA and American Academy of Neurology, that strongly recommend PFO closure in select populations. Although there have been many advancements in favour of PFO closure, this topic remains controversial. A lack of consensus among different guidelines creates ambiguity; therefore, the decision for PFO closure should involve shared decision-making with an interdisciplinary team.