The left atrial appendage (LAA) is now recognised as a key site of thromboembolic origin in atrial fibrillation (AF), acting as the primary source of around 90% of embolic strokes in non-valvular AF patients.1–4 An important therapeutic strategy for stroke prevention in AF targets the LAA and simultaneously offers the opportunity to circumvent long-term oral anticoagulants (OACs).2,5 The 2016 European Society of Cardiology (ESC) guidelines on AF management recommend that interventional LAA occlusion may be considered in patients at high stroke risk with contraindications to long-term OACs (Class IIb, level of evidence B).6 Surgical LAA occlusion or exclusion is also endorsed (Class IIb, level of evidence B) by the ESC for patients undergoing open-heart surgery or thoracoscopic AF surgery.6
Unmet needs in left atrial appendage occlusion
Approaches targeting occlusion of the LAA in non-valvular AF include surgical exclusion or occlusion, percutaneous catheter-based approaches and minimally invasive epicardial methods. To date, surgical LAA occlusion approaches such as simple suture ligation, endo- or epicardial oversewing and stapling exclusion have yielded disappointing results.5,7 In the majority of studies, surgical LAA techniques failed to demonstrate efficacy in stroke prevention and outcomes proved inconclusive, heterogenous or frankly negative as complete occlusion was not achieved.5,7 Undoubtedly, the most important reason for the lack of evidence supporting the various LAA occlusion techniques lies in the small number of patients studied and flawed study methods employed.
Percutaneous LAA closure devices provided the initial clinical evidence supporting LAA occlusion as a viable alternative strategy to OACs for stroke prevention in AF, however, shortcomings in this approach remain.4,5 Chief among the limitations are the high frequency of periprocedural complications and question marks surrounding the clinical impact of residual peri-device flow over the long term.2 A separate and significant drawback is the fact that patients need to continue anticoagulation, which is not necessarily the case with epicardial devices. Recognised procedure- and device-related complications associated with catheter occluder options include pericardial effusion or perforation, periprocedural stroke, device embolisation, bleeding and access-related vascular complications.1,5
The Watchman® device successfully demonstrated both non-inferiority and superiority over warfarin in the PROTECT-AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation; NCT00129545) trial for the combined outcome of stroke, cardiovascular death or other embolism.8 Yet paradoxically, there remains a dearth of clinical evidence for Watchman and similar percutaneous devices in OAC-ineligible patients – the specific target group for interventional LAA occlusion according to ESC guidelines. In PROTECT-AF, eligibility for long-term warfarin anticoagulation was actually a specific inclusion criteria for the trial and ~5% of patients were excluded at the screening stage due to inability to take warfarin.8 Patients treated with Watchman typically require a minimum of 6 weeks of post-procedural oral anticoagulation or dual antiplatelet therapy to facilitate endothelialisation of the device and reduce the risk of left atrial thrombi.3 Also, one consensus statement advises to continue single antiplatelet therapy indefinitely thereafter.9 Although the best strategy for post-procedural anticoagulation following Watchman placement is in flux, there is a trend away from using warfarin for 6 weeks. This presents a clear clinical dichotomy when considering percutaneous LAA occlusion devices in patients with true contraindications to OACs.3,4
The LAA has a complex and diverse anatomy with four broad morphological classifications – chicken wing (48%), cactus (30%), windsock (19%) and cauliflower (3%).2 Due to individual disparities in LAA morphology, patients can only be considered for a percutaneous LAA occlusion device if their cardiac anatomy is deemed suitable.4 A significant proportion will therefore be inherently ineligible for catheter closure due to anatomical limitations and underlying variability in their LAA morphology. Typically, the Watchman device can accommodate ~95% of LAA anatomy but use may be restricted based on the maximum LAA ostium diameter, the LAA depth and the threshold of shoulder protrusion into the left atrium, among other considerations.10 Particularly challenging anatomies for placement of percutaneous devices include proximal and severely sharp-angled chicken wing configuration, certain cactus configuration and limited depths.10 Published series clearly illustrate how anatomy-based selection for occluder devices is required to accommodate patients’ unique and individual anatomical variability.4 For example, of the 4,998 patients initially screened for inclusion in the PROTECT-AF trial of the Watchman device, 1,945 were excluded for ‘various clinical and echocardiographic reasons’.8
For patients not suitable for a percutaneous occluder approach, epicardial devices such as the AtriClip LAA Exclusion System (AtriCure, US) present an important, alternative option. AtriClip is applicable to all comers irrespective of their LAA morphology and allows for immediate cessation of anticoagulation, making it a suitable choice for patients who are ineligible for catheter closure due to anatomical restrictions or for patients with contraindications to OACs.3,4 The AtriClip can be placed irrespective of the LAA anatomy or left atrial dilatation and is not restricted by ostial size.11 Epicardial devices also benefit from an absence of interface between intracardiac foreign body and blood, potentially reducing the risk of recognised percutaneous complications such as thrombosis and infection. In addition, embolism is not an issue with epicardial devices.3
AtriClip clinical evidence
The AtriClip is the most widely used LAA exclusion device and comprises a single-use, sterile, repositionable, self-closing clip which is pre-loaded onto a single-use clip applicator.3 The clip itself is made from two parallel titanium tubes with elastic nitinol springs covered by a knitted braided polyester.2,3 The delivery system allows for redeployment/repositioning of the device as required, thereby facilitating optimal placement of the clip at the base of the LAA, permanently and totally occluding the appendage from circulating blood in the left atrium.2,3
Although viewed as a ‘surgical’ approach, deployment of the AtriClip device is a relatively simple and short procedure associated with minimal complications.3 Exclusion of the LAA can be achieved without injury to the heart or surrounding structures during concomitant open cardiac surgery.1 The AtriClip can also be deployed as a stand-alone, minimally invasive thoracoscopic procedure.2,3,5 Placement of the clip is non-traumatic and does not entail puncture of the atrial septum, groin or pericardial membrane. However, AtriClip placement is typically limited to surgeons performing the procedure. From a cost perspective, AtriClip compares favourably to both other LAA occlusion devices and long-term OACs.3
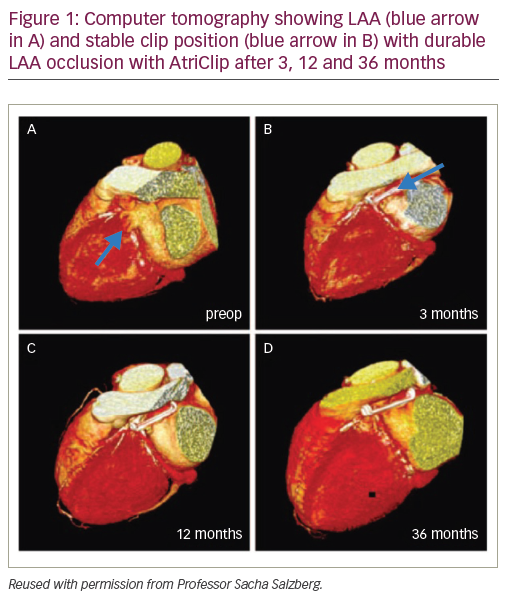
Evidence from several clinical studies supports the safety profile, efficacy and durability of epicardial LAA occlusion using the AtriClip device.1,4,5 Positive short-term safety and durability outcomes were attained with AtriClip in the prospective, non-randomised, multicentre EXCLUDE (Exclusion of Left Atrial Appendage with AtriClip Exclusion Device in Patients Undergoing Concomitant Cardiac Surgery; NCT00779857) trial.1 Of the 71 patients undergoing cardiac surgery, 70 had successful placement of the AtriClip device and there were no reported device-related adverse events. The LAA occlusion rate confirmed by both computed tomography (CT) angiography and transoesophageal echocardiography (TEE) exceeded 98%.1
The initial ‘first in man’ clinical study of AtriClip enrolled 40 patients with AF undergoing elective cardiac surgery and further validated the short-term feasibility and safety of epicardial LAA clipping.4 Clinical criteria excluding patients from receiving AtriClip in this trial included known LAA thrombus (evaluated by TEE), history of pericarditis, recent myocardial infarction and allergy to device components.4 Long-term data from this prospective device study after 3 years showed 100% durability and complete LAA occlusion confirmed by CT imaging.4 In total, 36 patients were followed up for a mean duration of 3.5 ± 0.5 years. Clip position remained stable over this 3.5-year period with no secondary dislocation evident on CT (Figure 1). Intracardiac thrombi did not occur and none of the LAA became reperfused: LAA occlusion was total and complete in 100% of patients. No strokes were documented during follow-up of this trial and anticoagulation could often be discontinued. From a safety perspective, no device-related complications or mortality occurred.4
A further study examined outcomes, including stroke risk impact, in 291 patients fitted with the AtriClip during open-heart surgery – 40 from the prospective trial cohort4 and 251 from a consecutive institutional registry.5 This mixed surgical cohort also included patients with valvular AF, in contrast to trials of Watchman that were carried out strictly in non-valvular AF.5 AtriClip delivery was performed in patients undergoing cardiac surgery (including isolated coronary artery bypass grafting, valve or combined procedures) with successful LAA occlusion controlled visually during the procedure and confirmed with simultaneous TEE, with subsequent repositioning as required. Long-term CT work-up in selected patients revealed LAA occlusion was 100% complete and durable 5 years or more post-implant, with no evidence of residual reperfusion or significant LAA stumps. In confirmation of the robust safety profile, no patients experienced device-related complications over the entire follow-up period post-implant, which ranged from 5.1 to 8.1 years. This study also provided the first circumstantial evidence of a significant reduction in stroke risk with AtriClip. The 166 patients who discontinued OACs after AtriClip deployment showed a relative stroke rate reduction of 87.5% (0.5/100 patient-years) compared with the expected rate for this CHA2DS2-VASc cohort (4.0/100 patient-years).5
As a stand-alone procedure, placement of the AtriClip device is not well recognised and available evidence remains limited. However, results from a recent case series of five patients ineligible for percutaneous treatment indicate that minimally invasive thoracoscopic closure of the LAA with AtriClip is a safe and feasible approach. In all patients, complete LAA closure was achieved without residual flow (confirmed by TEE) and with no complications or thrombi during 7.5 months of follow-up.11
Importantly, the AtriClip device not only completely excludes the LAA from the circulation but also affords complete electrical isolation, with no ongoing communication to the appendage.1,4 As an occlusion technique, clipping may therefore offer adjunctive electrophysiological benefits given the arrhythmogenic role of the LAA.4,12
From the available evidence, AtriClip appears to compare favourably with the Lariat catheter-based epicardial closure device, which is associated with a technical success rate of 93% and a lower procedural success of 83% due to major complications.10
Currently, AtriClip remains limited by a lack of supportive evidence from large-scale, prospective, randomised trials. Several studies are already underway to plug these data gaps, including a large randomised trial (NCT01561651) comparing surgical LAA occlusion with epicardial devices like AtriClip to no occlusion in over 4,500 AF patients scheduled for routine cardiac surgery, plus a prospective, multicentre study (NCT01997905) of stand-alone AtriClip LAA occlusion for non-valvular AF.5,13,14 As well as adding to the existing evidence base for the safety and durability of AtriClip, conclusive clinical data from these studies could serve to strengthen the guidelines positioning of LAA closure – both epicardial and percutaneous – as a valid stroke-prevention strategy.
Conclusions
There is a clear need for further optimisation in LAA occlusion and adoption of a tailored approach that takes into account patient-specific anatomical and morphological variability, as well as any future need for anticoagulation. Electrophysiologists, as well as cardiac surgeons, together in the AF heart team, have a key role to play in delivering this tailored treatment strategy, ensuring the optimal device and technique is selected for each individual AF patient to achieve the over-arching aim of safe, complete and durable LAA occlusion.
Catheter-based percutaneous approaches remain an important part of the electrophysiologists’ toolkit for LAA occlusion but fail to deliver a 100% solution due to inherent limitations. Minimally invasive epicardial LAA clipping therefore represents an important alternative therapeutic option for patients in AF who are not amenable to catheter closure and/or anticoagulation. The AtriClip epicardial device is suitable for all patients irrespective of their LAA morphology and has demonstrated long-term safety, durable LAA occlusion and a significant reduction in stroke risk.