The systemic amyloidoses are a group of heterogenous diseases, which are characterized by the deposition of insoluble amyloid fibrils within the extracellular matrix of multiple organs throughout the body. Amyloid fibrils are formed from the misfolding of amyloid precursor proteins (APPs) into insoluble, beta-pleated sheets that are resistant to proteolysis. They are histologically characterized by apple-green birefringence when stained with Congo red and examined under cross-polarized light.1,2 Transthyretin amyloidosis, better known as ATTR amyloidosis, occurs secondary to the misfolding of the transthyretin (TTR) protein. TTR is synthesized by the liver and circulates as a tetramer, acting as a physiological vehicle for the transportation of retinol and thyroxine. ATTR amyloid fibrillogenesis occurs when the stable tetrameric form of TTR becomes unstable and dissociates into monomers or is cleaved into fragments, the substrate from which the fibrils are derived. Disease occurs when the aggregation of amyloid fibrils disrupts the structure and function of an affected organ.3,4 The factors that determine organ tropism of amyloid deposits remain uncertain. However, electron microscopic examination of cardiac and nerve biopsies has demonstrated basement membrane thickening or reduplication.5 This is associated with the formation of advanced glycosylation end products, which are thought to potentially play a role in promoting amyloid formation and fibril accumilation.5
The formation of ATTR amyloid fibrils either occurs secondary to a pathological process that is associated with age-related homeostatic mechanisms, known as wild-type ATTR (ATTRwt) amyloid, or occurs secondary to an inherited single-point mutation in the TTR gene, which is known as hereditary ATTR or variant ATTR (ATTRv) amyloid.6 The clinical characteristics depend on the underlying inherited genetic mutation. ATTRwt amyloidosis predominantly affects males and is typically seen in patients over 65 years of age, although it may present as young as 50 years of age.7
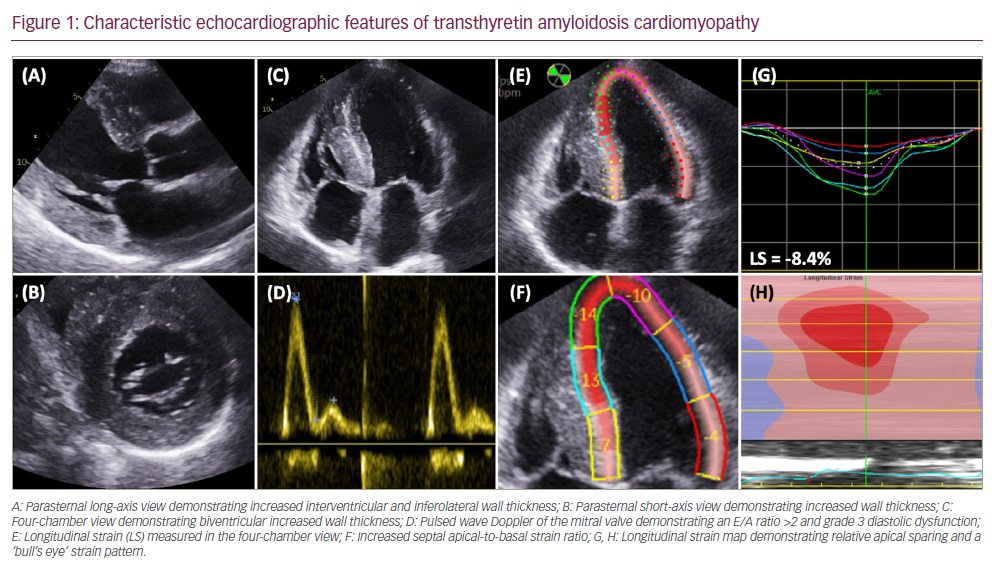
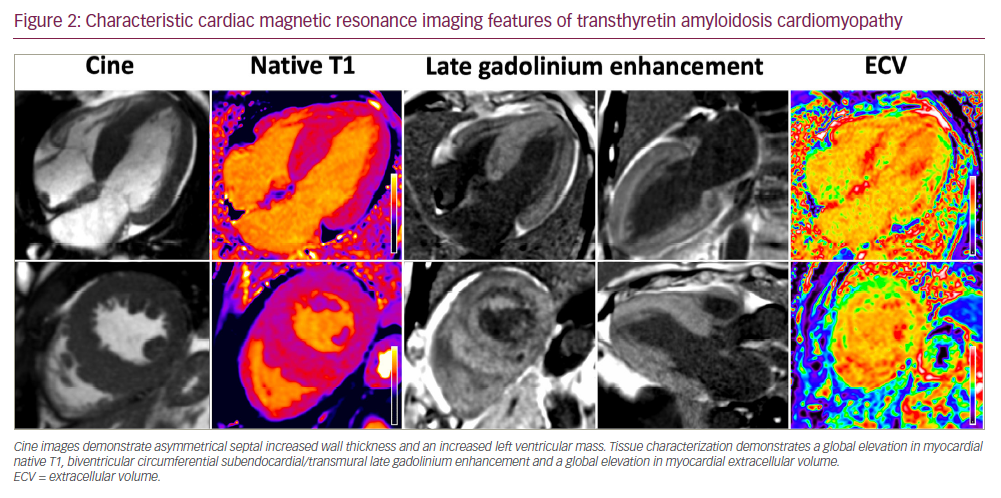
Myocardial deposition of ATTR fibrils causes an infiltrative and restrictive cardiomyopathy, typified by increased biventricular wall thickness and reduced myocardial compliance.8 Initially, stiffening of the myocardium results in increased left ventricular filling pressures and diastolic dysfunction. Evolution of the disease process leads to systolic impairment, which begins with longitudinal systolic dysfunction and is followed by radial systolic dysfunction. Amyloid infiltration begins in basal segments of the left ventricle and often results in a characteristic ‘bull’s eye’ strain pattern on echocardiography, with an increased septal apical-to-basal strain ratio and relative apical sparing (Figure 1). Cardiac amyloid infiltration also results in characteristic features on cardiac magnetic resonance (CMR) imaging, with elevated myocardial native T1, biventricular circumferential subendocardial/transmural late gadolinium enhancement and an elevated myocardial extracellular volume (ECV) (Figure 2).8–10 Cardiac manifestations of amyloid infiltration also include aortic stenosis, conduction disease and atrial fibrillation with an increased risk of thrombus formation.3,11 Extra-cardiac sites of amyloid infiltration can result in carpal tunnel syndrome, lumbar spine stenosis and tendinopathies.12–14 ATTRv amyloidosis may present at a younger age with a mixed clinical phenotype comprising ATTR cardiomyopathy (ATTR-CM) and a peripheral polyneuropathy (ATTR-PN). Patients presenting with polyneuropathy typically develop a progressive and debilitating, length-dependant, axonal sensory-motor peripheral polyneuropathy and/or autonomic neuropathy. The multisystem nature of ATTR amyloidosis is often best managed with multidisciplinary input from cardiologists, neurologists and orthopaedists.15 However, despite polyneuropathy causing severe debilitating symptoms, cardiac amyloid infiltration remains the main cause of mortality (Table 1).16,17
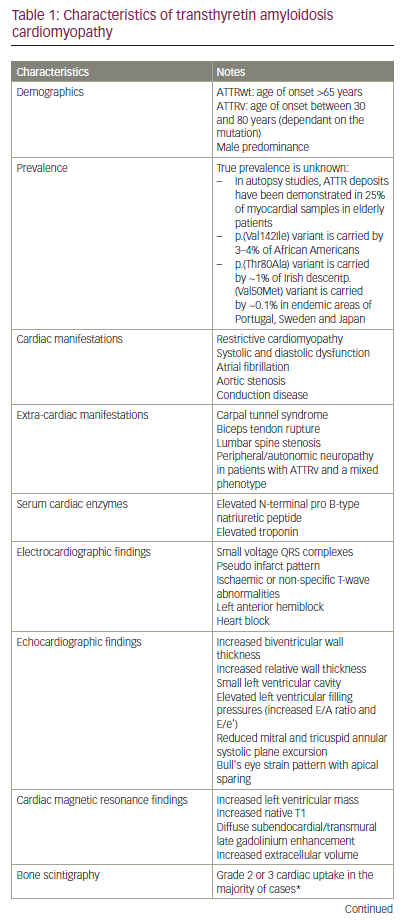
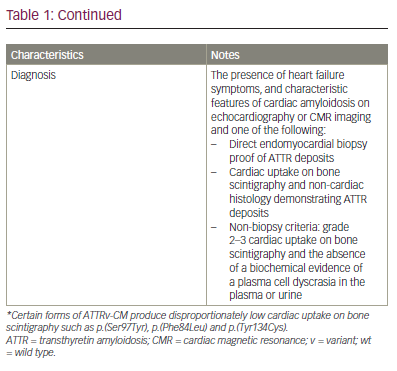
Traditionally, ATTR amyloidosis was considered to be a rare disease, but developments in diagnostics and increased awareness of the condition have resulted in a substantial increase in diagnoses over the last two decades. Increased recognition of ATTR amyloidosis has been driven in part by a non-biopsy diagnostic algorithm that utilizes the impressive sensitivity of bone scintigraphy for identification of cardiac ATTR amyloid deposits. In the absence of biochemical evidence such as a monoclonal gammopathy, the characteristic features of cardiac amyloidosis on echocardiography or CMR, combined with grade 2 or 3 cardiac uptake on bone scintigraphy, is diagnostic of ATTR-CM.18 This diagnostic algorithm has since been validated in several studies,19 including a large multicentre study that confirmed a high specificity and positive predictive value.20 In cases in which the non-biopsy criteria are not fulfilled, diagnosis is confirmed with either grade 2 or 3 cardiac uptake on bone scintigraphy and ATTR amyloid in an extra-cardiac biopsy, or direct endomyocardial biopsy proof of ATTR amyloid (Table 1).
The UK National Amyloidosis Centre (NAC) has been commissioned for the diagnosis and monitoring of amyloidosis. Data from the centre have demonstrated a dramatic increase in the number of ATTR-CM diagnoses over the last 20 years, from 35 cases between 2002 and 2006, to 968 cases between 2017 and 2021.21 A similar observation has been documented in Sweden, where the prevalence of ATTR-CM has increased from 1 per 100,000 to 5 per 100,000 over a 10-year period.22 However, the true prevalence of ATTR-CM remains unknown. With postmortem examinations revealing ATTR amyloid deposits in one-quarter of cardiac histological samples in elderly patients,23 and large population studies demonstrating certain TTR gene variants to be endemic in specific populations, the true prevalence is likely to be significantly higher than that reported in the medical literature.24
Before the availability of specific disease-modifying medicines, patients with p.(Val50Met) ATTRv-PN were successfully treated through liver transplantation. Following orthotopic liver transplantation, variant TTR is rapidly cleared from the systemic circulation due to its short plasma half-life, and replaced by wild-type TTR since there is no extra-hepatic source of circulating TTR. The halting of hepatic variant TTR production stops ATTRv fibril formation and the subsequent accumulation within the nervous system, and thus slows or prevents further deterioration of neuropathic symptoms. Success following liver transplantation supported the premise that reducing amyloidogenic TTR production could stabilize the disease process, and prevent further deterioration.25
The last decade has seen the development of multiple pharmacological therapeutic agents that can be used to treat ATTR amyloidosis. Initially, these agents were designed to induce targeted stabilization of the TTR tetramer, to prevent it from dissociating into amyloidogenic monomers or being cleaved into amyloidogenic fragments. This resulted in the development of tafamidis, a small molecule TTR stabilizer that was shown to reduce all-cause mortality in the seminal ATTR-ACT trial, and was subsequently licensed as the only available treatment of ATTR-CM.26 More recently, medicines have been developed with the aim of suppressing TTR production by specifically targeting and suppressing hepatic TTR synthesis.8 It is noteworthy that in all types of systemic amyloidosis in which reduction of the circulating APP (e.g. serum amyloid A protein in AA amyloidosis and monoclonal immunoglobulin light chain protein in AL amyloidosis) is achievable, reduction of the circulating APP has been associated with improved patient outcomes. Indeed, there is a strong correlation between the magnitude of ‘knockdown’ of the circulating APP and clinical outcomes.27–29 Studies with serial serum amyloid P scintigraphy,30 (a quantitative nuclear medicine technique that visualizes visceral amyloid deposits), and with serial ECV measurement by CMR,31 have shown that a substantial reduction of circulating APP concentration often leads to gradual amyloid regression from both the viscera and the heart. It is thus anticipated that deep reductions in circulating TTR will be associated with regression of amyloid and better outcomes in patients with ATTR amyloidosis. This treatment paradigm led to the development of patisiran, a first-generation small interfering RNA (siRNA) that targets and degrades the mRNA that codes for the TTR protein.32,33 This review will explore the use of patisiran for the treatment of ATTR-CM, and will also provide insights in future perspectives.
Patisiran
Mechanism of action and pharmacokinetics
Patisiran is the original siRNA that was specifically developed to treat patients with ATTR amyloidosis. siRNAs comprise double-stranded RNA molecules that bind to complementary mRNA molecules and cause degradation, hence interfering with protein synthesis. Following administration, siRNAs must be transported into the intracellular cytoplasm of the intended cells while evading breakdown from phagocytes and nucleases in the circulation, and also avoiding excretion.34–36 Patisiran is formulated as an encapsulated lipid nanoparticle that protects the siRNA molecule. The lipid nanoparticle is coated in apolipoprotein E, which binds to the low-density lipoprotein on the hepatocyte membrane to ensure targeted delivery. Once released into the hepatocyte intracellular cytoplasm, the double-stranded siRNA molecule divides into two single-stranded RNA molecules, which bind to a genetically preserved sequence in the 3′ untranslated portion of the complementary wild-type or variant TTR mRNA. The binding process triggers Argonaute slicer protein activation, which subsequently degrades the mRNA, thereby inhibiting TTR synthesis.32,33
Patisiran is administered as an intravenous infusion of 0.3 mg/kg every 3 weeks, with a single administration achieving a maximum reduction in serum TTR concentration of 88%, and a steady plasma level being achieved by 24 weeks.37 Patisiran is metabolized into nucleotides and has an elimination half-life of 3 ± 2 days, with less than 1% being excreted unchanged in the urine. Mild or moderate renal impairment (chronic kidney disease stage 1–3) and mild hepatic impairment do not affect the pharmacokinetics of patisiran or its ability to induce TTR knockdown, and patisiran does not interfere with cytochrome P450 activity, which is responsible for the metabolism of many small molecule drugs (Table 2).32
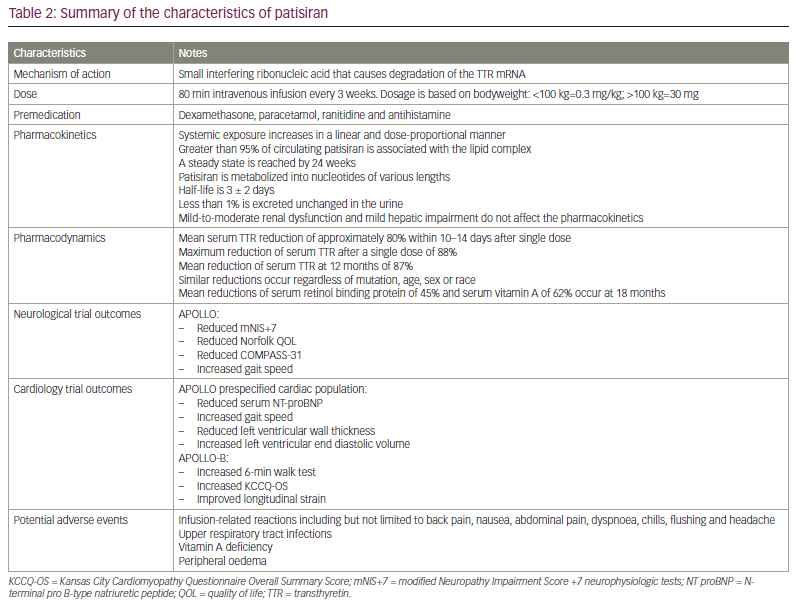
Clinical trials
Following a successful phase I clinical trial,37 a phase II clinical trial of 29 patients with ATTRv-PN demonstrated that after two doses of patisiran the mean reduction in serum TTR from baseline was 85%, and the maximum reduction was 96%.38 In a 24-month open-label extension of 27 patients, 11 of whom had concomitant cardiac involvement, patisiran treatment halted the progression of neurological disease and stabilized cardiac disease, with there being no significant changes in serum cardiac enzymes or echocardiographic parameters.39
The APOLLO phase III trial was a double-blind, placebo-controlled, randomized study of 225 patients with ATTRv-PN, and was designed to evaluate the safety and efficacy of patisiran.29 After 18 months of treatment, there was a significant improvement from baseline in the modified Neuropathy Impairment Score +7 neurological tests (mNIS+7), Norfolk Quality of Life Questionnaire-Diabetic Neuropathy (Norfolk QOL-DN) score and gait speed in the group treated with patisiran, compared with the placebo group.29 Patients treated with patisiran demonstrated a significant improvement compared with those on placebo across all components of the Composite Autonomic Symptom Scale 31, which is designed to assess the burden of autonomic neuropathy.40 Following the success of the APOLLO trial, patisiran was approved for the treatment of ATTRv-PN by both the European Commission and the United States Food and Drug Administration in 2018.32,33
Cardiac outcomes
To assess the efficacy of patisiran in patients with ATTR-CM accompanying ATTR-PN (i.e. ATTR-mixed), a post-hoc analysis was carried out on a subset of 126 patients enrolled in the APOLLO trial, who were considered to have cardiac involvement based on prespecified criteria of a left ventricular wall thickness ≥13 mm in the absence of hypertension or significant aortic valve disease.41 Treatment with patisiran was associated with a reduced mean left ventricular wall thickness and relative wall thickness at 18 months compared with placebo. Patisiran treatment also resulted in an increased left ventricular end diastolic volume and improved global longitudinal strain and cardiac output. The patisiran group had a significant reduction in serum N-terminal pro B-type natriuretic peptide (NT-proBNP) and a significant increase in 10-metre walk test gait speed when compared with patients in the placebo group. The favourable changes in both cardiac structure and function were associated with a lower combined event rate of all-cause hospitalization and mortality in the treatment group compared with the placebo group. However, this post-hoc analysis does have significant limitations. The prespecified definition of cardiac involvement does not adhere with published diagnostic recommendations, and despite being sensitive, it lacks specificity. A relatively small cohort of patients were included, with only 36 patients in the placebo arm, and due to the post-hoc design, the analysis was unblinded. Lastly, despite a significantly lower all-cause hospitalization and mortality rate in the treatment group, there was no significant difference in cardiovascular hospitalizations and mortality, which raises the possibility that observed differences could be due to treatment of the underlying polyneuropathy rather than cardiomyopathy. Nevertheless, this analysis was the first to suggest that patisiran may halt the progression of cardiomyopathy in ATTR amyloidosis; however, the findings require confirmation in a randomized controlled trial in patients with ATTR-CM.41
The observations above were reinforced by a small but important study in 16 patients with ATTR-mixed, treated with patisiran and diflunisal.42 Treatment was well tolerated and achieved >80% knockdown in serum TTR in 82% of cases, which was associated in some patients with a significant reduction in CMR-derived ECV and NT-proBNP, and an increase in 6-minute walk test (6MWT) distance following 1 year of treatment. Baseline and follow-up bone scintigraphy revealed a reduction in cardiac uptake following patisiran treatment. However, although the reduction in cardiac uptake on bone scintigraphy undeniably demonstrated a favourable biological effect of patisiran, the kinetics and dynamics of radiotracers binding to the myocardium, bones and soft tissues may fluctuate, and therefore variations in any of these organs will influence both the visual appearance and mathematical calculation of proportionate myocardial radiotracer uptake. Therefore, reduced myocardial uptake on bone scintigraphy should always be supported by improvements in other measures of cardiac function, such as either serum cardiac enzymes or alternative cardiac imaging modalities, before the observed changes are attributed exclusively to a reduction in the burden of cardiac amyloid.42
The APOLLO-B trial is a phase III randomized placebo-controlled, double-blind study that enrolled 360 patients to evaluate the efficacy and safety of patisiran treatment in patients with ATTR-CM.43,44 The APOLLO-B trial enrolled patients with both wild-type and variant ATTR-CM who had clinical evidence of heart failure and an elevated NT-proBNP between 300 ng/L and 8,500 ng/L. Patients were randomized 1:1 to either patisiran or placebo. Patisiran treatment resulted in sustained reductions in serum TTR, with a mean knockdown of 87% at 12 months. Patisiran demonstrated significant clinical benefit in functional capacity as measured by the change in 6MWT results at 12 months compared with placebo, with the decline in 6MWT in the patisiran group being similar to a typical age-related decline seen in healthy adults. A prespecified sensitivity analysis using a mixed-effects model for repeated measures confirmed the robustness of these findings and demonstrated a significant mean difference in 6MWT distance at 12 months of 18 metres between the patisiran and placebo groups. Patisiran treatment resulted in a significant and favourable difference in the change from baseline in Kansas City Cardiomyopathy Questionnaire Overall Summary score (KCCQ-OS) at 12 months compared with placebo. However, there was no difference in the time to first event (all-cause hospitalization, urgent heart failure visits or death). Despite there being no statistically significant differences in mortality between the two groups, which may in part reflect the short study duration, the data were numerically in favour of patisiran (4 patients [2%] versus 10 patients [6%]). The efficacy and safety of patisiran treatment in patients with ATTR-CM will continue to be assessed during the open-label extension. It is noteworthy that although the APOLLO-B results have been presented at international conferences, the full manuscript is yet to be published, and therefore more detail is required before formal conclusions can be drawn.43,44
Safety
Patisiran is prescribed with premedication consisting of an antihistamine, a corticosteroid, paracetamol and ranitidine to reduce the risk of patients developing infusion-related reactions (Table 2). During the phase I trial of patisiran, 21% of participants had mild or moderate infusion-related reactions. All mild reactions resolved spontaneously, and moderate reactions resolved after temporarily stopping the infusion and then continuing the infusion at a slower rate.37 Continued use of patisiran for over 18 months was associated with a 62% reduction in serum vitamin A levels, which reflects the physiological function of TTR as a carrier for retinol-binding protein that, in turn, transports vitamin A. Therefore, all patients prescribed patisiran are also routinely prescribed oral vitamin A supplementation (2,500 IU per day) as prophylaxis.32 In the APOLLO phase III trial there were no significant differences in the frequency of adverse events between patients on patisiran treatment and those in the placebo group, and importantly, there were no reports of eye complications. Infusion-related reactions and peripheral oedema occurred more commonly in the patisiran group, but there was a low incidence of serious adverse events. There were seven deaths in the patisiran group, none of which were related to treatment as all were thought to be consistent with the natural progression of ATTRv amyloidosis.29 In the APOLLO-B trial, patisiran was well tolerated and the vast majority of adverse events were either mild or moderate in severity. Infusion-related reactions, arthralgia and muscle spasms were more common in patients treated with patisiran than in the placebo group. There were four deaths in the patisiran group, none of which were deemed to be related to treatment.44
Comparison between patisiran and tafamidis
Tafamidis remains the only licensed treatment for ATTR-CM, and was approved following the ATTR-ACT trial that demonstrated a reduction in the composite hierarchical primary endpoint of all-cause mortality and cardiovascular hospitalizations, as well as a lower rate of decline in 6MWT distance and KCCQ-OS score compared with placebo.26 Despite patisiran treatment resulting in favourable changes across a range of metrics, it has not yet been shown to influence cardiovascular outcomes. Although it is possible that these disparities relate to different drug mechanisms, they are also likely influenced by the differences in study populations and study design. APOLLO-B was a 12-month study and the primary endpoint was change in 6MWT, rather than cardiovascular events. ATTR-ACT was a 30-month study and the primary endpoint was a hierarchical composite of all-cause mortality followed by cardiovascular hospitalizations. Baseline patient characteristics indicate more advanced cardiac disease among ATTR-ACT study participants than those in the APOLLO-B trial, and consequently the event rate in ATTR-ACT was higher. A larger population would have been required in APOLLO-B to ensure adequate power to demonstrate a difference in cardiac events. These data also raise questions regarding the efficacy of individual treatments at different stages of the disease course, and more data are needed to clarify the relative treatment benefits in different disease stages.21
Tafamidis and patisiran differ in their route of administration. Tafamids is administrated orally and is thus convenient; patisiran is an intravenous infusion that requires premedication and co-prescription of prophylactic vitamin A. Both have a good safety profile, although a smaller proportion of patients in the treatment arm of ATTR-ACT reported adverse events than in APOLLO and APOLLO-B, where the majority of events were infusion-related reactions. However, in the absence of any head-to-head trials, comparisons between these different treatments are, by definition, flawed.26,29,43,44
Patisiran is able to achieve >80% serum TTR knockdown in the majority of patients.45 However, this leaves a significant proportion of circulating TTR that could misfold into ATTR fibrils. It is conceivable that simultaneously reducing TTR production and inducing TTR stabilization could be synergistic and further improve patient outcomes. However, large-scale trials would be needed to assess whether combination therapy has any added benefit.45
Monitoring the cardiac response to patisiran
Following promising trial results there has been an increasing interest in the use of patisiran to treat ATTR-CM, and at present there is an unmet clinical need to develop standardized measures of the cardiac response to treatment. Advancements in cardiac imaging, combined with increased awareness among clinicians, has resulted in patients being diagnosed earlier in the disease process. This is evident by a shorter duration of heart failure and neuropathic symptoms prior to diagnosis, and a milder NAC disease stage at the time of diagnosis.21 This has translated into improved survival and a reduced event rate in the ATTR-CM population, which will invariably have an influence on the design of future clinical trials, with a greater number of patients being recruited and for longer follow-up durations in order to ensure adequate power.21 Such changes will substantially increase the cost of clinical trials, and the question remains as to whether cardiac biomarkers or imaging measures can be leveraged as surrogates to facilitate a safe but more cost-effective assessment of novel medicines.
NT-proBNP has long been utilized in the heart failure population to assess the response to heart failure medications, and also in AL amyloidosis to monitor treatment response.46 However, NT-proBNP fluctuations represent the final common pathway of multiple interlinked mechanisms such as ventricular remodelling, renal dysfunction, fluid status and neurohormonal activation;47 hence, these changes do not necessarily reflect the change in cardiac amyloid burden.48 High-sensitivity troponins are widely used in the assessment of multiple acute cardiac conditions,49 and contribute to risk stratification in cardiac AL amyloidosis.50 Due to their high sensitivity, even small fluctuations reflect changes in disease activity.49 However, their use in monitoring treatment response in ATTR-CM requires further exploration and validation.
The search for a clinical biomarker that can easily measure the cardiac response to patisiran has led to increasing interest in the use of multimodality cardiac imaging. Improvements in various echocardiographic parameters have been reported following treatment with patisiran.41 However, echocardiographic measures are subject to high levels of intra- and inter-observer variability, which represents a significant limitation when only small changes in echocardiographic parameters are expected.51 The emergence of data science has the potential to significantly improve precision and overcome these obstacles.8 Various studies have utilized convolutional neural networks in multiple different modalities of cardiovascular imaging, and to date, all have yielded encouraging results.52,53 The automation of measurements is associated with a significant improvement in precision, and may facilitate a more accurate assessment of the cardiac response to treatment with patisiran.52,54
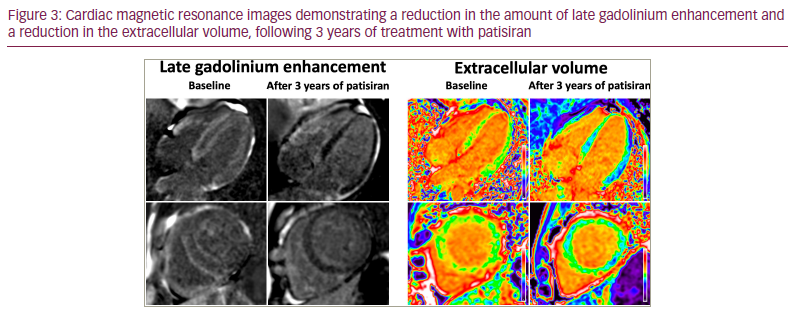
CMR with multi-parametric mapping has emerged as an essential tool for the assessment of ATTR-CM. Amyloidosis is the exemplar interstitial disease, with amyloid accumulation resulting in expansion of the extracellular matrix. Therefore, myocardial ECV quantification acts as a powerful surrogate measure of the cardiac amyloid burden.4,55,56 In patients with cardiac AL amyloidosis, changes in ECV following treatment predate any change in structural or functional parameters and accurately predict survival, even after adjusting for traditional predictors, such as change in NT-proBNP and longitudinal strain57 .31 At present, only one small study of patients with ATTR-CM has demonstrated ECV regression following treatment with patisiran, but if these results were to be reproduced on a larger scale, ECV mapping could become more widely utilized to assess the cardiac response to patisiran (Figure 3).42,45
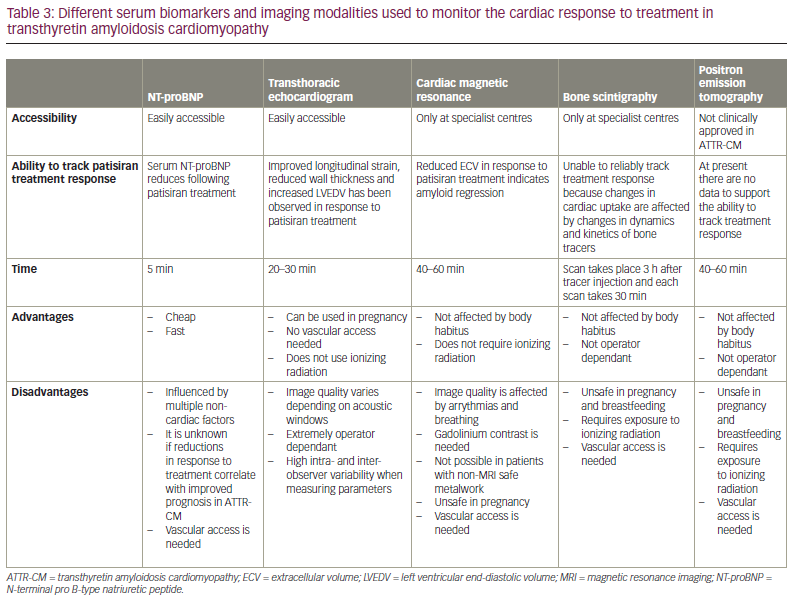
Although reduced cardiac uptake on bone scintigraphy has been reported following treatment with patisiran, changes in myocardial radiotracer uptake are also heavily influenced by changes in the dynamics and kinetics of bone tracers, and therefore bone scintigraphy alone cannot be used as a reliable measure of the cardiac response at present.42 The use of positron emission tomography and various novel tracers has shown diagnostic potential in ATTR-CM, but at present there is a lack of robust data supporting their use in tracking treatment response (Table 3).58–64
Long-term safety of patisiran
Although short- to medium-term patisiran-induced TTR knockdown appears safe, studies with prolonged follow-up are required to establish whether there are any long-term side effects. The physiological role of the TTR protein was previously thought to be limited to the transportation of thyroxine and vitamin A. However, various in vitro and in vivo studies suggest that TTR may have additional roles within the central nervous system. Animal studies have demonstrated that 5-month-old mice with complete TTR knockdown developed significant memory impairment compared with age-matched mice,65 and TTR deficiency in rats lead to accelerated age-related decline in cognitive performance.66 These findings have fuelled the hypothesis that the TTR protein may have a role in delaying Alzheimer’s-related neurodegeneration, a disease process characterized by the accumulation of amyloid-β plaques within the brain. In vitro studies demonstrated that TTR sequesters amyloid-β when added to cerebrospinal fluid,67 and mouse models have demonstrated hippocampal upregulation of TTR in response to the formation of hippocampal amyloid-β plaques, suggesting a possible neuroprotective response.68 Subsequent anti-TTR antibody infusion led to augmented amyloid-β plaque development, loss of neurones and neuronal apoptosis.69 In mouse models, surgical occlusion of the middle cerebral artery led to a significantly larger infarct area and greater degree of cerebral oedema in those with complete TTR depletion,70 while subsequent incubation of the hippocampal neurones with recombinant TTR was associated with improved neurite growth.71 The possible neuroprotective effect of TTR in cerebrovascular ischaemia has also been observed in humans, with a large observational cohort study of patients with cerebral infarction demonstrating that serum TTR levels represented an independent predictor of good outcomes.72
Importantly, there has been no evidence to suggest cognitive decline or abnormality in patients receiving patisiran treatment continuously for over 5 years.45 Furthermore, patisiran is unable to cross the blood–brain barrier and therefore does not interfere directly with TTR synthesis in the choroid plexus and retinal pigment epithelium, which are responsible for the production of TTR within the cerebrospinal fluid.73
Future perspectives
Advances in siRNA conjugate delivery platforms assisted the development of vutrisiran, which is a 3-monthly subcutaneous injection that is administered without the need for predication. The HELIOS-A trial randomized patients with ATTRv-PN to vutrisiran or patisiran, and compared outcomes with an external placebo group from the APOLLO trial.74 Vutrisiran treatment achieved similar serum TTR knockdown and was non-inferior to patisiran, resulting in improved mNIS+7 and Norfolk QOL-DN scores when compared with placebo. It has since been approved for the treatment of ATTRv-PN.74,75 The ongoing HELIOS-B trial (ClinicalTrials.gov identifier: NCT04153149) aims to assess the efficacy of vutrisiran for the treatment of ATTR-CM, and if successful will potentially offer a convenient alternative to patisiran.76
Conclusion
ATTR-CM remains a progressive and ultimately fatal disease. Reduction of the respective circulating APP in all types of amyloidosis in which it is possible, has long been known to be associated with improved patient outcomes. Similarly, recent studies have demonstrated that reducing the circulating TTR concentration with patisiran results in improvement in a wide range of cardiac and neurological disease measures, including cardiac enzymes, functional capacity, multiple cardiac imaging-based parameters, neurological scores and quality of life. These exciting results will potentially result in expansion of the patisiran license to include ATTR-CM, and increase the armamentarium of disease-modifying treatments available for this aggressive, but treatable, cardiomyopathy. While there have been no deleterious clinical consequences in the short to medium term of patisiran-induced TTR depletion, long-term effects remain to be determined.