Heart failure (HF) and atrial fibrillation (AF) are inexorably linked. They frequently coexist and share common risk factors, including ageing, hypertension, diabetes, obesity, sleep apnoea and coronary disease.1–4 Over half of patients with HF develop AF at some point,5 and when AF occurs, it is associated with an increase in total mortality of up to 40%.6 In addition, HF admissions complicated by AF are associated with higher mortality and repeat admission.7 Among patients with AF, HF has been associated with a doubling of mortality regardless of whether the HF was pre-existing or concurrently diagnosed the same day.8 In addition, annual direct and indirect costs of HF alone are high, estimated at $30 billion in the USA.9 With stakes this high, managing AF in the setting of HF has been an area of intense focus.
Rhythm control through pharmacological means has been tested but has repeatedly met with limited success in patients with HF with reduced ejection fraction (HFrEF).10–13 Dofetilide and amiodarone have both been used in high-quality randomized control trials to clarify the impact of sinus rhythm on the poor outcomes associated with HFrEF. In patients with HF and ejection fraction (EF) ≤35%, rhythm control failed to show a survival benefit, and HF hospitalizations were mixed – reduced in the DIAMOND-CHF (Danish Investigations of Arrhythmia and Mortality on Dofetilide in Congestive Heart Failure) study but increased in the AF-CHF study (Atrial fibrillation and congestive heart failure trial; ClinicalTrials.gov Identifier: NCT00597077).12,13 Therapy for HFrEF has changed considerably since these studies were performed.14–16 In fact, the RACE 3 study (Routine versus aggressive upstream rhythm control for prevention of early atrial fibrillation in heart failure; ClinicalTrials.gov identifier: NCT00877643), using more contemporary HF therapy, showed improvement in the rates of sinus rhythm without using anti-arrhythmic agents.17 Nonetheless, the rates of long-term sinus rhythm in earlier trials reflect the best therapy and best monitoring for AF at the time.12,13 Compared with more contemporary monitoring, these older trials have overestimated the rates of sinus rhythm; therefore, the impact of the therapy are more limited. The limited success of pharmacological rhythm control in these trials may have also limited the ability to demonstrate any advantage. Certainly, this is one explanation for why the results of ablation-based rhythm control trials appear to be different.
Catheter ablation for the long-term maintenance of sinus rhythm has slowly evolved and improved over the last two decades. As the success rates improved, the impact of sinus rhythm in patients with HF eventually came back into focus. Specifically, it was hypothesized that, if sinus rhythm could be achieved in sufficient numbers of patients with HF through ablation, perhaps a benefit could finally be exposed. In this article, we review the growing evidence that catheter ablation-based rhythm control results in improved quality of life (QoL), as well as EF, 6-minute walk distance and B-type natriuretic peptide (BNP) levels. Further, recent trials have shown improved all-cause mortality and HF events. Is it time to incorporate catheter ablation into the mainstay of HFrEF patients who develop AF?
Endpoints that are surrogates for mortality
With the evolution of catheter ablation for AF, investigators readdressed whether sinus rhythm could improve outcomes in patients with HFrEF over a rate control strategy18–23 or, in a smaller number of studies, rhythm control using anti-arrhythmic drugs.23,24 Most studies chose as endpoints those that have been independently associated with improved survival in pharmacological HF trials including EF, 6-minute walk distance, VO2 max and QoL. Muddying the waters somewhat is the therapy in the control arm; five studies used rate control, one study allowed rate and rhythm control pragmatically, and one used amiodarone exclusively as rhythm control.18–23 Nonetheless, individually and when combined in meta-analyses, statistically and clinically important improvements in EF of a magnitude associated with improvements in mortality in pharmacological trials (~5–7%), 6-minute walk distance, VO2 max and QoL were seen (Table 1).18,19,21–30
It is worth noting that the AMICA trial (Atrial fibrillation management in congestive heart failure with ablation; ClinicalTrials.gov identifier: NCT00652522) was included in none of the most recent systematic reviews, likely because it was stopped early due to futility as the improvement in EF in the ablation group (8.8%, 95% CI 5.8–11.9) was similar to the best medical therapy group comprising rate or rhythm control (7.3%, 95% CI 4.3–10.3; p=0.36).31 Sinus rhythm was seen in 73.5% of the patients in the ablation group and 50.0% of those in the best medical therapy group. Interestingly, in the AATAC trial (Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device; ClinicalTrials.gov identifier: NCT00652522), where sinus rhythm was achieved using amiodarone, minimal EF improvement was seen (8.1% versus 6.2%).24 These two studies stand out compared with those in which rate control was used substantially or mandated in the control group.
Heart failure hospitalizations and mortality revisited in the ablation era
Given the improvements in the surrogate endpoints noted above, improvements in HF hospitalizations and all-cause mortality might be expected for patients with HFrEF undergoing AF ablation. A small number of studies looking at these “harder” outcomes are summarized in Table 1.
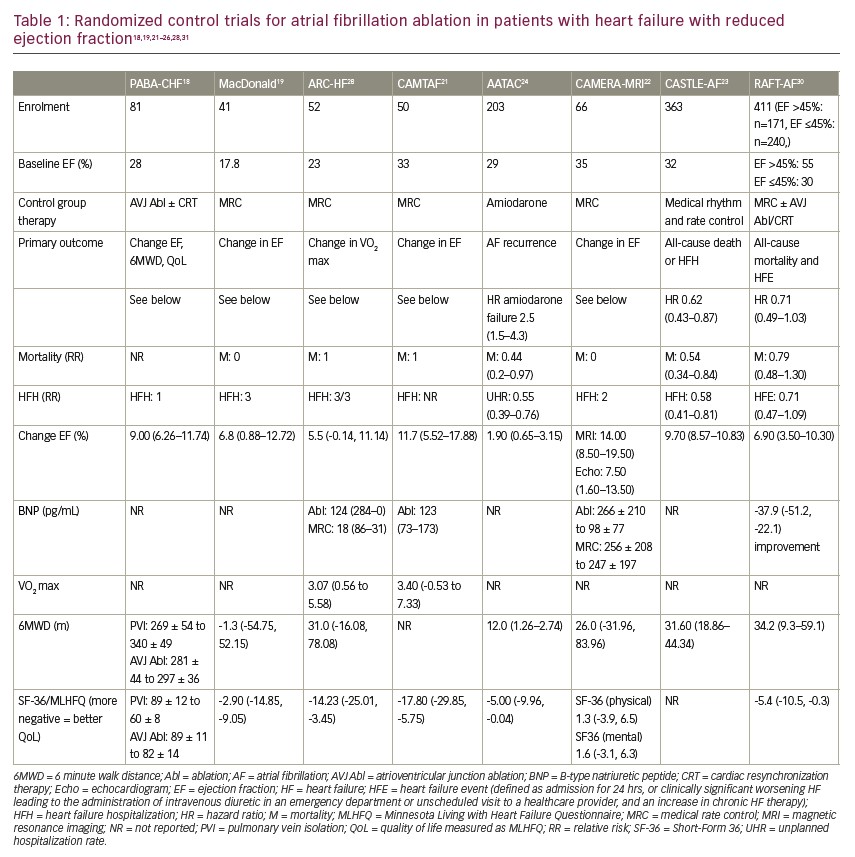
The AATAC study first suggested that sinus rhythm achieved by catheter ablation was superior to amiodarone.24 It randomized 203 patients with persistent AF, left ventricular (LV) dysfunction (mean EF 30%) and New York Heart Association (NYHA) class II–III symptoms. Sinus rhythm was maintained in 70% (95% confidence interval [CI] 60–78) of patients undergoing 1.4 ± 0.6 catheter ablation procedures over 2 years compared with 34% (95% CI 25–44) of those randomized to amiodarone (log-rank p<0.001). A doubling of the rate of sinus rhythm with ablation was impressive, given that atrial electrograms from dual chamber devices were used to monitor for AF. The ablation arm had greater improvements in EF (8.1±4 [median 8.3%] versus 6.2±5.0 [median 5.0%]; p=0.02), 6-minute walk distance (22±41 [median 19 m] versus 10±37 [median 6 m]; p=0.02) and QoL (11±19 [median 10] versus 6±17 [median 5.0]; p=0.04) compared with the control arm. In addition, the ablation arm had fewer unplanned hospitalizations (31% versus 57%; p<0.001) and fewer deaths (8 versus 18; relative risk 0.44, 95% CI -0.20, 0.96; p=0.037; number needed to treat: 10 patients) compared with subjects receiving amiodarone, although the numbers were quite small. The AATAC study first suggested that the maintenance of sinus rhythm using catheter ablation could result in better clinical outcomes, including mortality.
The CASTLE-AF trial (Catheter ablation vs. sandard conventional therapy in patients with LV dysfunction and AF; ClinicalTrials.gov identifier: NCT00643188) sought to confirm these findings in a similar population but longer follow-up.23 All patients had implantable cardioverter–defibrillator or cardiac resynchronization therapy defibrillator devices for LV dysfunction (EF 32%) and class II–IV HF symptoms. The trial was pragmatic in that patients were randomized to pharmacological rate or rhythm control in one arm versus catheter ablation, and patients who had failed or were unable or unwilling to take an anti-arrhythmic drug were included. While sinus rhythm was encouraged, rate control, when used, targeted a ventricular rate of 60–80 beats per minute (bpm) at rest and 90–115 bpm during moderate exercise.
Five weeks after randomization and a medical run-in phase, 179 patients received catheter ablation and 184 medical therapy, be it rate or rhythm control. After 37.8 months, patients undergoing catheter ablation had an impressive 38% fewer deaths from any cause or unplanned HF hospitalization compared with those in the medical therapy group (primary endpoint). This included a 47% reduction in deaths, a 44% reduction in unplanned HF hospitalization alone (hazard ratio for event 0.56, 95% CI 0.37–0.83; p=0.004) and a 51% reduction in cardiovascular deaths compared with the medical therapy group (hazard ratio 0.49, 95% CI 0.29–0.84; p=0.009) (all secondary endpoints). Rates of worsening HF appeared to separate in the two arms as early as 1 year according to the Kaplan–Meier curves, whereas it took up to 3 years to show a difference in the rates of death.
Like in the AATAC study, AF burden was determined using continuous monitoring by atrial electrograms. AF burden fell from 50% to under 30% in the ablation arm but remained unchanged with medical therapy. The mean EF improved by 8% in those undergoing ablation, and two-thirds of these patients had an EF increase to 35% or greater. EF remained unchanged for the most part in the medical therapy arm. There were some significant limitations to the CASTLE-AF study; as pointed out by a recent review, “there were significant imbalances across the treatment groups at randomization; 20% of all randomized patients were not included in the primary analysis; follow-up was twice as likely to be missing in the ablation than in the control group; and the trial was stopped for futility and achieved only 70% of the planned number of primary end point events”.31
The RAFT-AF study is the latest study to be published (Randomized ablation-based rhythm-control versus rate-control trial in patients with heart failure and atrial fibrillation; ClinicalTrials.gov Identifier: NCT01420393).30 It followed a rigorous PROBE (Prospective Randomized Open-label study with Blinded Outcomes) design and randomized 411 patients with high-burden paroxysmal AF (more than four episodes in 6 months) or persistent AF (less than 3 years), NYHA class II–III HF, and elevated N-terminal pro BNP (NT-proBNP) levels to receive either ablation-based rhythm control or rate control. Unlike previous studies, the RAFT-AF study included patients with impaired LV (EF ≤45%, n=240) and preserved LV (EF >45%, n=171) function. The primary outcome was a composite of all-cause mortality and all HF events, with a minimum follow-up of 2 years. Patients were stratified by EF, and a priori subgroup analyses were planned.
At follow up visits, sinus rhythm was recorded in 86% of patients in the ablation-based rhythm control group over 24 months compared with 13% in the rate control group. Rate control was excellent, with a mean heart rate of 75 bpm at rest; 60 patients required atrioventricular node ablation and biventricular pacemaker implantation. A high rate of optimal medical therapy was used, including oral anticoagulation use in 95% of patients.
Compared with rate control, ablation-based rhythm control resulted in improvements in LVEF (10.1 ± 1.2% versus 3.8 ± 1.2%; p=0.017), 6-minute walk distance (44.9 ± 9.1 metres versus 27.5 ± 9.7 metres, p=0.025), NT-proBNP (mean change -77.1% versus -39.2%; p<0.0001), and HF-specific QoL as measured by the Minnesota Living with Heart Failure Questionnaire (least squares mean difference of -5.4, 95% CI -10.5, -0.3; p=0.0036) and AF-specific QoL measured as AF Effect on Quality-of-life Questionnaire score (least squares mean difference of 6.2, 95% CI 1.7–10.7; p=0.0005). All-cause mortality or HF event occurred in 50 of 214 (23.4%) patients in the ablation-based rhythm control group compared with 64 of 197 (32.5%) patients in the rate control group (hazard ratio [HR] 0.71, 95% CI 0.49–1.03; p=0.066).
The group with EF ≤45% showed more pronounced improvements in 6-minute walk distance, QoL and EF, with an impressive 15% improvement at 24 months. In this subgroup with impaired LV function, all-cause mortality or HF event occurred in 28 of 124 (22.6%) patients in the ablation-based rhythm control group compared with 43 of 116 (37.1%) patients in the rate control group (HR 0.63, 95% CI 0.39–1.02; p=0.059). This effect size, estimated by the HR of catheter-ablation rhythm control over rate control, is virtually identical to that of the CASTLE-AF study (51 patients [28.5%] versus 82 patients [44.6%]; HR 0.62; 95% CI 0.43 to 0.87; p=0.007), although the mortality rate in the control group was higher in the CASTLE-AF study.
The primary outcome in RAFT-AF was numerically lower in the ablation arm but just missed statistical difference. Unfortunately, the impact of ablation on the primary outcome took considerable time to manifest as it did with prior ablation studies. The interim analysis may have underestimated the full impact of the intervention as the difference in the primary outcome was realized only after 18 months of follow-up. This was also seen in the CASTLE-AF study, where mortality curves did not separate for 2 years.23 Certainly, all the secondary outcomes ,including NT-proBNP, EF and 6-minute walk distance, associated with improvements in HF-related death and hospitalization were statistically improved. Although the primary analysis of RAFT-AF was neutral, ablation may result in a decrease in HF hospitalization and mortality in patients with HFrEF when put in the context of the totality of the evidence, including surrogate endpoints as well as the results of AATAC and CASTLE-AF.
Heart failure with preserved ejection fraction
Very few randomized data exist examining the effect of the catheter ablation of AF in patients with a clinical diagnosis of HF with preserved EF (HFpEF). Moreover, the diagnosis of HFpEF has not been universally established using biomarker, haemodynamic or imaging methods.32 Nonetheless, two recent meta-analyses combined data from both retrospective and prospective observational studies to investigate the AF recurrence in those with HFpEF undergoing ablation.33,34 The first combined data from six studies and 1,505 patients to compare outcomes in those with HFpEF versus those with HFrEF. Similar AF recurrence rates were seen at 1 year.23 No difference in hospitalization was seen, but as expected, mortality was lower in the HFpEF group compared with the HFrEF group.
The second meta-analysis combined seven observational studies representing data from 1,696 patients.34 Four studies compared ablation results in those with and without HFpEF. Three studies compared outcomes in patients with HFpEF undergoing ablation versus medical therapy. Rates of sinus rhythm after ablation, fluoroscopy and procedure times were similar in patients with HFpEF compared to those without HFpEF. Ablation improved the maintenance of sinus rhythm and reduced rehospitalization for HF in patients undergoing ablation compared with medical therapy. No mortality differences were seen. Two further meta-analyses showed very similar results.35,36
In the large CABANA trial (Catheter ablation vs anti-arrhythmic drug therapy for atrial fibrillation; ClinicalTrials.gov identifier: NCT00911508), one-third of participants (778 patients) had NYHA II–IV symptoms at baseline.37,38 Of these patients, 15% had a history of HF. The median EF was 55%. Only 8% of them had EF ≤35%, suggesting that most patients had HFpEF. The ablation subgroup, in this subgroup, resulted in a 44% reduction in AF recurrence compared with the control group (rate or thythim control therapy). An intention-to-treat analysis showed a 36% reduction in the primary endpoint, a composite of death, disabling stroke, serious bleeding or cardiac arrest (HR 0.64, 95% CI 0.41–0.99) and a 43% reduction in all-cause mortality (HR 0.57, 95% CI 0.33–0.96) in the ablation group compared with drug therapy over a median 48.5-month follow-up. These patients had preserved LV function and reported symptoms of HF, but the diagnosis of HFpEF was not clearly established using biomarkers, haemodynamic parameters or structural abnormalities.32 Furthermore, the presence of HF was not stratified at randomization, so the post hoc analyses of this subgroup need to be interpreted with caution.
The RAFT-AF study was the first to provide high-quality data on the impact of ablation-based rhythm control on patients with HFpEF.30 The group with EF >45% (HFpEF) receiving ablation showed statistically improved NT-proBNP measures similar in magnitude to the subgroup with reduced LV function.31 This translated into a small, clinically insignificant improvement in EF (3% at 24 months) but no improvements in the primary endpoint, AF-specific or HF-specific QoL or 6-minute walk distance. A number of other small single-centre studies have shown improvements in NT-pro BNP and small improvements in EF.39–42 Changes in EF are, by definition, limited by near -normal baseline values, by definition. Perhaps, future large-scale, high-quality trials will demonstrate an improvement in HF hospitalizations and mortality in the HFpEF population. At present, the best evidence suggests that do not appreciably benefit form ablation-based rhythm control.
One further interesting finding from the RAFT-AF study is worthy of comment. Analysis by AF type, which was stratified at the time of randomization, demonstrated a greater effect in the paroxysmal and early persistent AF group (<7 days) for ablation-based rhythm control (HR 0.24, 95% CI 0.08–0.70; interaction p=0.171) than in the persistent AF (duration >7 days but <1 year) (HR 0.68, 95% CI 0.43–1.09) and long-term persistent AF (duration >1 year) (HR 1.13, 95% CI 0.50–2.57) groups. This is not the first study to suggest better outcomes for ablation when performed early in a patient’s course. The EARLY-AF study (Early aggressive invasive intervention for atrial fibrillation; ClinicalTrials.gov identifier: NCT02825979) demonstrated a 50% reduction in AF recurrence after ablation as first-line therapy. The mean percentage time in AF in this group was 0% (interquartile range, 0–0.08).43 Likewise, in the STOP AF First study (STOP AF First: Cryoballoon catheter ablation in an antiarrhythmic drug naive paroxysmal atrial fibrillation; ClinicalTrials.gov identifier: NCT03118518), the percentage of patients with treatment success at 12 months was 74.6% (95% CI 65.0–82.0) in the ablation group and 45.0% (95% CI 34.6–54.7) in the drug-therapy group.443 It appears that the response to ablation is better early in the course of AF, regardless of the underlying substrate.
Conclusions
There is accumulating evidence that patients with HFrEF benefit greatly from sinus rhythm when achieved using catheter ablation. Improvements in EF, QoL, 6-minute walk distance, HF hospitalizations and mortality have been demonstrated in more than one randomized study. This appears to be the case whether the control group received medical therapy, including amiodarone, or rate control. This does not appear to be the case for HFpEF, where ablation improved NT-proBNP but none of the other important outcomes.
Perhaps sinus rhythm provides differing benefit for HFrEF compared with HFpEF. Clearly, sinus rhythm provides good rate control. However, it also eliminates diastolic irregularity. To the extent that on-going diastolic irregularity in rate control may contribute to or exacerbate LV dysfunction in a manner similar to premature ventricular contraction-induced cardiomyopathy, sinus rhythm may correct this, maximizing improvements in EF.45 Currently, there is insufficient evidence to recommend catheter ablation for AF in patients with HFpEF to reduce mortality, HF hospitalizations or improve QoL. Further clinical research and large-scale well-conducted clinical trials are needed to fully elucidate the impact of catheter ablation for AF in patients with HFpEF and HFrEF. ❑