As clinicians and academics, we have at least three reasons for developing a simple stepwise algorithm for the management of atrial fibrillation (AF) with rapid ventricular response (RVR) in the emergency department (ED). First, AF is the most common rhythm disorder encountered by physicians, including in the acute cardiovascular care setting of the ED.1 The presentation of AF in the ED can be an innocent bystander or the primary or secondary cause of the patient’s critical condition.2,3 Second, physicians are often faced with a choice between rhythm control and rate control management, with the consideration of several variables in outpatients or patients with chronic AF. Moreover, different variables may need to be considered in the emergency setting, such as haemodynamic stability, which can be confusing when making decisions.4 Third, physicians have limited time to consider these variables and make immediate decisions in the ED. Several major guidelines have partially reviewed haemodynamic instability, rate or rhythm control management, cardioversion, anticoagulation, pre-excited AF, and CHA2DS2-VASc scores (congestive heart failure, hypertension, age >75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years, sex category [females]). However, only a few of these sources has synthesized and compiled these topics into practical steps. Therefore, we have developed a stepwise algorithm to address the challenges of treating patients with AF in the ED by summarizing and incorporating the latest updates and guidelines.2,4–8 In order to conduct a comprehensive and practical review, a collaboration was carried out with two major cardiovascular experts in acute cardiovascular care and electrophysiology to create this review. Through this algorithm, we hope to create the best stepwise approach based on the latest evidence-based medicine to simplify and speed up the work of physicians in the management of AF in the ED. We present and review each step to make it easier to understand and apply the practical steps (Figure 1).
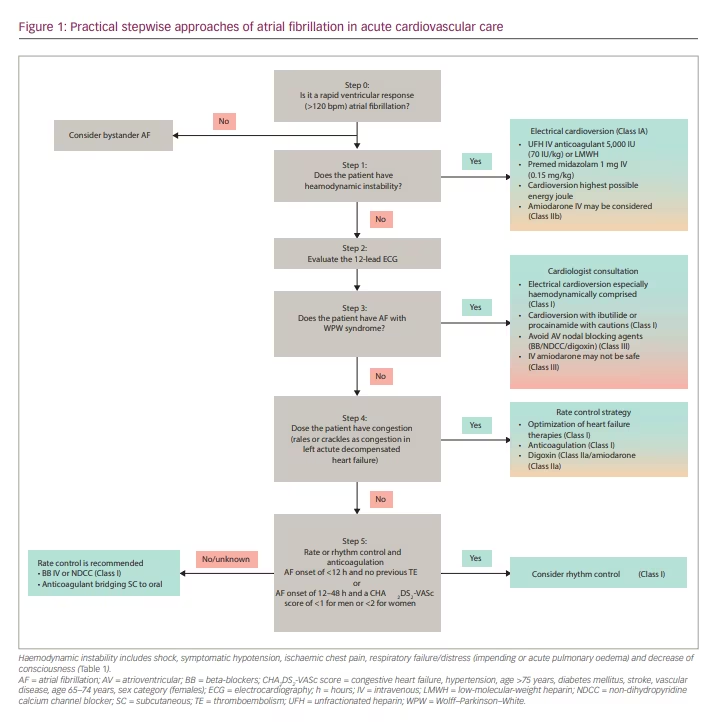
Step 0: Is it a rapid ventricular response to atrial fibrillation?
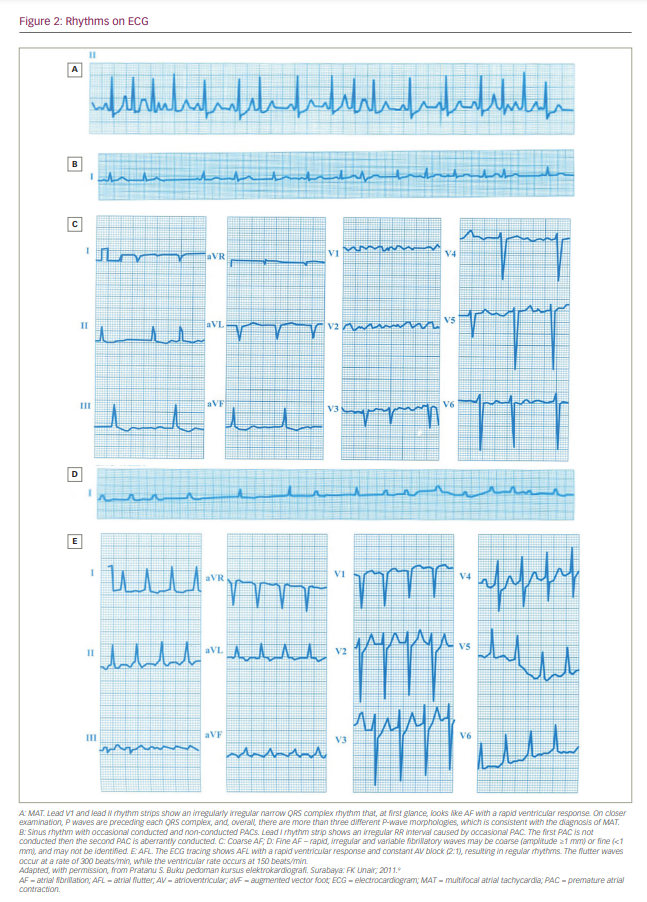
The initial step is to perform a 12-lead electrocardiogram (ECG) or to recognize a single ECG tracing on the patient’s monitor for the diagnosis of AF. This is important because the physician must confirm the diagnosis of AF so that further stepwise approaches can be applied. The diagnosis of AF requires a standard 12-lead ECG or a single-lead ECG tracing >30 seconds that shows a heart rhythm with no discernible repeating P waves and irregular RR intervals when atrioventricular (AV) conduction is not impaired.5 However, there are two things to keep in mind when diagnosing AF. First, it is necessary to ensure that the ECG rhythm is an AF and not other rhythms that are similar to AF (Figure 2).9 The AF rhythm should be distinguished from other rhythms with irregular RR intervals, such as multifocal atrial tachycardia, wandering pacemaker or high-degree AV block with variable ratios.10,11 Second, it is necessary to evaluate the ventricular response in AF. Rapid ventricular rate and the lack of atrial contribution can impair ventricular filling, cardiac output and coronary perfusion, thus increasing myocardial oxygen demand. This condition is frequently observed in patients with severe acute heart failure (AHF), on-going myocardial ischaemia or hypotension. On the other hand, the moderate or slow ventricular response of AF rarely causes haemodynamic instability. For example, AF with slow or moderate ventricular response can be considered an innocent bystander in AHF, and an investigation of other causes of AHF should be carried out. In this situation, the treatment of AHF and its underlying causes are more critical than AF treatment.2,3
In AF with an RVR >120 beats/min, further evaluation using the stepwise approach is necessary. The threshold for RVR causing haemodynamic instability may vary in each guideline. The Advanced Cardiovascular Life Support guidelines use an RVR value of >150 beats/min, a common threshold for most tachyarrhythmias causing haemodynamic instability. In comparison, the position paper of the Acute Cardiac Care Association of the European Society of Cardiology (ESC) and European the Heart Rhythm Association position statement use a value as low as 120 beats/min. Accordingly, we selected the threshold value that may cause haemodynamic instability, >120 beats/min, based on clinical experience and the ESC position statement.2,4,6
Step 1: Does the patient have haemodynamic instability?
All physicians and guideline recommendations agree that the first step in managing a patient with AF RVR is to evaluate haemodynamic instability. Several objective parameters, such as saturation, capillary refill time, blood pressure, urine output and the Glasgow Coma Scale, can be evaluated in the ED.12 However, in clinical practice, differences in the subjective clinical judgement of physicians are observed in determining haemodynamic instability. Therefore, before proceeding to the next approach, it is necessary to have a common understanding of haemodynamic instability (Table 1).13–18
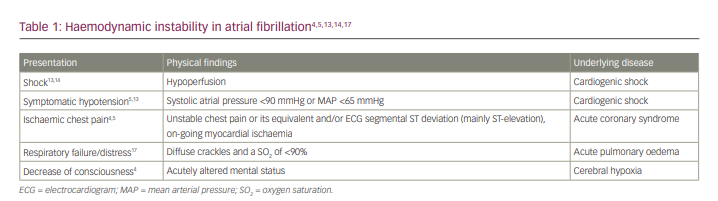
- Shock. Although many references generally describe shock without specifying the aetiology, it is important to emphasize that the instability, in this case, is cardiogenic shock due to AF leading to hypoperfusion (cold and clammy skin, cyanosis, urine output <0.5 mL/kg/h, altered mental state, disorientation and confusion). Therefore, it is critical to identify the aetiology of shock. Aggressive therapy based on the underlying mechanism of the shock becomes more critical in shock with other aetiologies such as distributive, hypovolemic or obstructive shock.13 Several diagnostic tools, such as point-of-care ultrasound or echocardiography, can be used to help identify the type of shock.14 In this condition, hyperlactatemia is typically present (>1.5 mmol/L), indicating abnormal cellular oxygen metabolism.13
- Hypotension. In adults, systolic arterial pressure is <90 mmHg or the mean arterial pressure is <65 mmHg, with associated tachycardia.13
- Acute coronary syndrome. Chest pain that meets the criteria for haemodynamic instability includes ischaemic chest pain or discomfort.4,5 When acute coronary syndrome is diagnosed according to the ESC 2020 criteria for non-ST-segment elevation acute coronary syndrome and the 2017 ESC for ST-segment elevation myocardial infarction, either with symptoms of unstable or equivalent chest pain or ECG changes (especially ST-segment elevation), it can be classified as haemodynamic instability or as impending haemodynamic instability. Acute coronary syndrome accompanied by AF, especially new-onset AF will exacerbate the imbalance of oxygen supply and demand, necessitating an immediate rhythm control with electrical cardioversion and revascularization when necessary.15,16
- AHF. Some consensus includes any type of AHF as haemodynamic instability, while others include acute pulmonary oedema as haemodynamic instability. Based on the latest ESC 2021 guidelines for acute and chronic heart failure, there are four clinical manifestations of AHF, including acute decompensated heart failure, acute pulmonary oedema, isolated right ventricular failure and cardiogenic shock. From these differences, AHF with impaired perfusion or cardiogenic shock and acute pulmonary oedema were considered as haemodynamic instability.17 We agree to the inclusion of pulmonary congestion as haemodynamic instability when pulmonary oedema or impending respiratory failure is present (oxygen saturation <90% in room air and crackles >50% of lung fields) and to its exclusion when there are only signs of right-sided heart failure, such as increased jugular venous pressure, leg oedema or hepatojugular reflex.17,18 In left heart failure without pulmonary oedema, we evaluate the presence and severity of pulmonary congestion. A patient with crackles in >50% of lung fields is more likely to have pulmonary oedema, and electrical cardioversion can be considered. In contrast, the finding of crackles in <1/3 of lung fields will be discussed in step 4.17 Considering that AHF is a dynamic condition, the ED physician may perform cardioversion when the condition of the patient worsens by considering the benefits and risks of thromboembolic events.
Electrical cardioversion in the emergency setting should be initiated without delay in severely compromised patients. To obtain better output in electrical cardioversion, it is preferable to directly use the highest energy employing by a biphasic defibrillator over an energy escalation strategy whenever possible. Biphasic defibrillators are the standard because of their superior effect compared with monophasic defibrillators.19 Both anterolateral and anteroposterior positions can be considered; if one fails, then the other approach can be applied.20
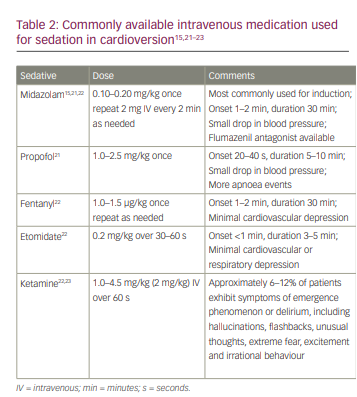
Prior to cardioversion, it is necessary to administer heparin 70 IU/kg intravenous bolus (max 5000 IU), if there are no contraindications (active bleeding or suspicion of intracranial haemorrhage), and sedation with midazolam (0.15 mg/kg).15 Heparin is chosen because of its rapid onset of action, availability (including its antidotes) and pharmacoeconomic advantages. In addition, parenteral low-molecular-weight heparins enoxaparin (1 mg/kg twice daily), dalteparin (200 IU/kg daily or 100 IU/kg twice daily, not to exceed 1800 IU daily) and tinzaparin (175 IU/kg once daily) can be used as an alternative.2 Midazolam is preferred because it is easier to titrate, works for longer, has a lower risk of respiratory depression and has retrograde amnesia.15 Other sedatives that may be used are shown in Table 2.15,21–23 The target level of sedation is usually moderate to deep.24 Patients with no improvement in haemodynamic stability and immediate recurrence of AF during evaluation for 1 minute after the first electrical cardioversion may undergo a second cardioversion with antiarrhythmic drugs pre-treatment such as amiodarone and adequate ventilation, as this may improve the efficacy of the electrical cardioversion.25,26
Step 2: Evaluate the 12-lead electrocardiogram
Following the exclusion of haemodynamic instability, the next step is to review the patient’s current and past 12-lead ECGs, if obtainable (patients may have had prior examinations). Although it may seem simple, detailed interpretations of the ECG will determine the next steps. An ECG assessment can be performed using the following steps.
- Confirm the diagnosis of AF on ECG strips and rule out other possible irregular rhythms.10,11
- Evaluate the presence of Wolff–Parkinson–White (WPW) syndrome in AF, especially in AF with wide QRS complexes.27
- Evaluate ST-segment changes primarily to exclude ST-segment elevation myocardial infarction.28
- Evaluate current or previous ECG signs of structural remodelling and potential causes of AF that may increase the risk of thrombus, such as chamber enlargement in hypertrophic cardiomyopathy, poor R progression and pathological Q wave in ischaemic cardiomyopathy, and generalized low voltage in amyloid cardiomyopathy.29–33
- Evaluate for signs of electrolyte disturbances, especially potassium disturbances, in patients taking amiodarone or digoxin.34,35
- Evaluate signs of digitalis intoxication, especially in patients taking digoxin.34
Simultaneously, take the history of the patient, and correlate it with the ECG interpretation. To conclude, three important points to conclude at the end of this step are the confirmation of the diagnosis of AF, evaluation of the WPW syndrome and prediction of the onset of AF.
Step 3: Does the patient have atrial fibrillation with Wolff–Parkinson–White syndrome?
Groups of AF patients with pre-excitation or WPW syndrome require special attention. WPW syndrome is estimated to occur in 0.1–0.3% of the population and is most commonly observed in the age group of 20–24 years.36 AF is not uncommon in patients with the WPW syndrome, with an incidence of 11.5–39.0%.37 AF accompanied by WPW may be fatal because it can produce an RVR with non-decremental conduction through the accessory pathway. The ventricular response generated through the accessory pathway can reach more than 300 beats/min and may degenerate to ventricular fibrillation. This mechanism is considered a common cause of sudden cardiac death in patients with WPW syndrome, with a mortality rate up to 0.6% per year.27 Differentiating pre-excited AF with polymorphic ventricular tachycardia (VT) and AF with aberrant ventricular conduction may be challenging. Here are a few key features that can help in differentiating between the two conditions.27
- AF with WPW syndrome should be suspected in tachycardia with wide and irregular QRS complexes. Several important features lead to the diagnosis of AF with WPW syndromes, such as an irregular rhythm, RVR (too fast for conduction through the AV node) and the wide-bizarre QRS complex. Occasionally, a narrow QRS may be seen, indicating conduction through the AV node. Careful interpretation of the ECG must be confirmed within the clinical context. The probability of AF with WPW syndrome is increased in younger patients (<50 years) with a previous history of palpitations, rapid heart rate, syncope or a documented history of WPW syndrome. However, the rapid ventricular rate and wide QRS complex are poor differentiators of AF with WPW syndrome from other wide complex tachyarrhythmias. Meanwhile, irregular rate and variation of bizarre QRS complex morphologies suggest AF with WPW syndrome.
- The ECG features of polymorphic VT are similar to those of AF with WPW syndrome. Polymorphic VT has wide QRS complexes with a fast ventricular rate (150–300 beats/min), variable RR intervals and frequently changing QRS complexes. Torsades de pointes is a subtype of polymorphic VT that occurs in the setting of QT prolongation with undulating baselines that distinguishes it from AF with the WPW syndrome, which usually has a stable baseline with no alteration in the polarity of the QRS complex.
- AF with aberrant ventricular conduction is observed when the impulse from AF is conducted to the ventricle with a pre-existing bundle branch block or rate-dependent bundle branch block. The ECG shows irregular broad complex tachycardia with monotonous QRS configuration, unlike AF with WPW syndrome with variable QRS configuration.
In conclusion, AF in young patients presenting to the ED with a history of palpitations or tachyarrhythmias, ECG features with an irregular heart rate, and the wide and unusual or altered QRS complex is suggestive of a diagnosis of AF with WPW syndrome. ECG criteria can also be used for older patients with caution because older patients may have other dysrhythmic events such as supraventricular tachycardia with aberrant ventricular conduction, monomorphic VT and polymorphic VT.27 Consultation with a cardiologist is advised when the diagnosis is uncertain.
The management of unstable AF with WPW syndrome is immediate electrical cardioversion. In stable AF with WPW syndrome, pharmacological cardioversion can be attempted using intravenous ibutilide (1 mg [0.01 mg/kg for patients <60 kg] over 10 minutes);27,38 in contrast, procainamide (30 mg/min, maximal dose 17 mg/kg), propafenone (1.5–2.0 mg/kg over 10 min) and flecainide (2 mg/kg over 10 min) should be used with caution,due to their effect on the AV node.39–41 Pharmacological cardioversion should be done with continuous monitoring and access to electrical cardioversion. AF with WPW syndrome should not be treated with drugs that prolong conduction through the AV node, such as adenosine, beta-blockers, digoxin or non-dihydropyridine calcium channel antagonists (NDCCs). In addition, the administration of intravenous amiodarone in AF with WPW syndrome is potentially harmful.27,36,42 After the emergency condition is resolved, patients with a history of supraventricular arrhythmias with WPW or patients with symptomatic WPW syndrome are advised to undergo catheter ablation.36,42
Step 4: Does the patient have congestion (crackles as congestion in left acute decompensated heart failure)?
By step 4, haemodynamic instability, including AHF with impaired perfusion, acute pulmonary oedema and WPW syndrome, should have been ruled out. AF is both a cause and consequence of heart failure, leading to systolic and diastolic dysfunction. On the other hand, the neurohormonal and anatomical changes in heart failure make the development and progression of AF much more likely. It is important to distinguish the course of the disease between permanent AF that progressed to AHF and chronic heart failure that subsequently developed new-onset AF, as rhythm control in the former settings might be difficult to achieve and maintain, whereas heart failure therapy in the latter setting requires priority treatment.2,43 Pulmonary congestion can be quickly identified by the presence of crackles (51% sensitivity and 81% specificity) and orthopnoea (44% sensitivity and 89% specificity).44 Meanwhile, the S3 heart sound in AF is difficult to evaluate, especially in RVR.
In patients identified as ‘wet’ (at step 4 with crackles ≤1/3 of the lung fields) and ‘warm’, rate control agents with amiodarone or digoxin may be given (Table 3).2,5,45–48 The use of drugs that have negative inotropic effects, such as NDCCs, should be avoided.2 However, when bedside echocardiography or information on ejection fraction is available, beta-blockers and NDCCs are safe for patients with heart failure with preserved left ventricular ejection fraction; in contrast, beta-blockers may be used in those with heart failure and reduced left ventricular ejection fraction even with crackles ≤1/3 of the lung fields.45,49,50
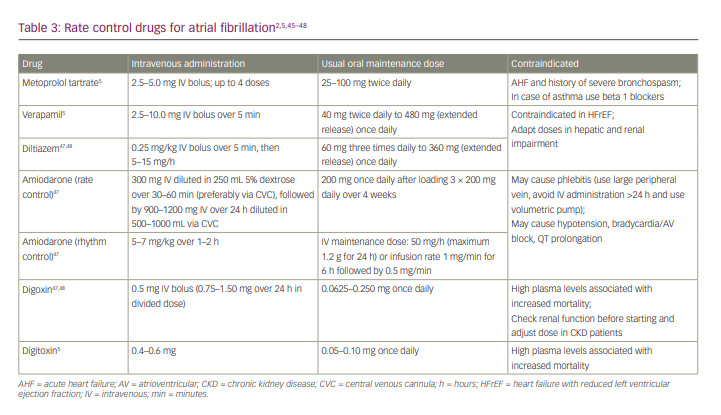
As the recommended rate control agent, digoxin is preferred over amiodarone for the following reasons. First, there is no evidence to suggest a superiority between digoxin and amiodarone in the acute cardiovascular care setting.51,52 Second, considering the safety of the access route, digoxin is safe to administer via a peripheral intravenous line, whereas the administration of amiodarone with a concentration of 1.5–2.0 mg/mL or continuous infusion over 1 hour carries a risk of phlebitis.53,54 Third, amiodarone carries a risk of accidental rhythm conversion because the dose for rate control may overlap with the dose for rhythm control. Fourth, amiodarone has a long half-life, whereas rate control may be used only for a short period, that is, until AHF resolves and a beta-blocker can be administered. In addition, the second dose of digoxin may be given after evaluating the ventricular response 2–6 hours after the first dose, along with the evaluation of AHF therapy, particularly urine production after diuretic administration.55,56
In addition to rate-controlling agents with digoxin or amiodarone, anticoagulant therapy may be administered using vitamin K antagonists or direct oral anticoagulants (DOACs) (heart failure is also a thromboembolic risk for AF).57,58
Step 5: Rate or rhythm control management and anticoagulation
In this fifth step, after confirming that the patient does not have pulmonary congestion and perfusion disorders, the remaining problem to treat is AF with the RVR. Unlike in the previous step, rate control and rhythm control management may be considered in this step. The decision between rate or rhythm control should be discussed with the patients, considering the risk and benefit of each approach. Rate control may be a reasonable choice in asymptomatic patients with AF, particularly with recurrent and long-standing AF.59,60 The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial and the Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation (RACE) trial showed that rate control is not inferior to and perhaps has a better outcome than rhythm control in patients with long-standing AF.61,62 Another consideration related to the choice of rate control is the proarrhythmic effect of antiarrhythmic drugs. This side effect can cause drug intolerance and increase rehospitalization rates.61,63 Further considerations are the appreciable rate of recurrent AF and the frequent crossover to rate-control strategy. Recurrence of AF in rhythm control may be detectable in about 20–60% of patients within 1 year.64 The risk of AF recurrence on rhythm control is increased in patients with hypertension, left atrial enlargement and an AF duration of >1 year or heart failure.65 Physicians can perform pharmacological rate control using beta-blockers, NDCCs, digoxin, amiodarone or combination therapy (Table 3). However, beta-blockers and NDCCs are preferred over digoxin and amiodarone in this step because of their rapid onset of action and effectiveness even at exercise.45–48 On the other hand, recent-onset AF, failure of rate control, younger age, tachycardia-mediated cardiomyopathy, minimal atrial remodelling, no or few comorbidities, and AF precipitated by temporary event or acute illness are factors favouring rhythm control.5 The EAST-AFNET 4 trial (Early treatment of atrial fibrillation for stroke prevention trial [EAST]; ClinicalTrials.gov identifier: NCT01288352) which enrolled 2789 patients with early AF diagnosed within a year and at high risk for cardiovascular complications and assigned to early rhythm control with antiarrhythmic drugs or ablation or to usual care, found that early rhythm control therapy resulted in slightly improved survival and a lower adverse cardiovascular outcome.65 It is important to note that the majority of the patients in this study were pharmacologically controlled with antiarrhythmic drugs, while ablation constituted less than 20%.66
According to the ESC AF 2020 guidelines, there are three main things to consider in determining the appropriate cardioversion management, including the onset of AF, history of thromboembolism and CHA2DS2-VASc score.5 In AF with an onset of <12 hours without a history of thromboembolism or AF with an onset of 12–48 hours and a CHA2DS2-VASc score of <1 for men or <2 for women, cardioversion can be performed within the first 48 hours of the onset of AF. In these cases, the physician can use pharmacological or electrical cardioversion with pre-anticoagulation. The choice between pharmacological and electrical cardioversion should be based on the availability of drugs and health personnel, hospital facilities and shared decision-making between the patient and the physician. Pharmacological cardioversion is less effective than electrical cardioversion, but this approach allows physicians to attend to other patients during the drug infusion and frequently avoids the risk of sedation. When pharmacological cardioversion fails, the physician can then switch to electrical cardioversion. This drug–shock treatment is more effective than electrical cardioversion alone (successful conversion: 96% versus 92%, respectively).26 Another strategy to consider is a wait-and-see approach (initial rate control and delayed cardioversion if needed). The RACE 7 ACWAS trial (Acute cardioversion versus wait and see approach for symptomatic atrial fibrillation in the emergency department [RACE 7 ACWAS]; ClinicalTrials.gov identifier: NCT02248753) showed that the wait-and-see approach is as safe as and not inferior to immediate cardioversion of paroxysmal AF, which often spontaneously resolves within 24 hours.67
Elective cardioversion can be performed in cases of AF with an onset of >48 hours, AF with unknown onset, AF with an onset of 12–48 hours and a CHA2DS2-VASc score of >2 for men or >3 for women, AF with a history of thromboembolism, AF with a moderate-to-severe mitral stenosis, or AF with prosthetic mechanical heart valves. Elective cardioversion, either electrical or pharmacological, can be given after >3 weeks of effective anticoagulation with DOACs or within <3 weeks of DOAC administration with transoesophageal echocardiography that excludes a thrombus in the left atrium or left atrial appendage.5
Patients who undergo rhythm control management using electrical or pharmacological cardioversion at steps 1, 3 or 5 should receive 4 weeks of DOACs regardless of the CHA2DS2-VASc score because nearly all thromboembolic events with cardioversion occur within 10 days of the procedure.25 After 4 weeks of DOACs, the decision on long-term oral anticoagulant treatment is determined by the presence of risk factors for stroke. Anticoagulant treatment is optional for AF patients with onset <24 hours and at very low risk of stroke with a CHA2DS2-VASc score of 0 in men or 1 in women. Meanwhile, patients with rate control management at steps 1, 3 or 5 and CHA2DS2-VASc scores ≥1 in men or ≥2 in women should receive long-term oral anticoagulation.5
Conclusion
We have described some practical steps for the management of rapid AF in the ED. This approach may help in the quick and precise management of rapid AF. However, it does not necessarily replace previous rapid AF recommendations, such as the Advanced Cardiovascular Life Support guidelines, the ESC guidelines and the Acute Cardiac Care Association/European Heart Rhythm Association position statement but provides physicians with additional considerations for making wise decisions.