Over the past two decades, the field of transcatheter aortic valve replacement (TAVR) has experienced remarkable advancement. Initially approved for patients with high and prohibitive surgical risk, TAVR has progressively extended its indications to all patients, regardless of surgical risk. Beyond the expansion of indications, substantial efforts have been dedicated to refining and optimizing both the transcatheter heart valve (THV) and delivery catheter designs. These iterative improvements aim to enhance procedural success rates, minimize complications such as paravalvular leak (PVL) and permanent pacemaker implantation (PPI), and improve overall patient outcomes. The aim of this review is to provide an overview of the development and current status of the Navitor™ TAVR system, with a scope limited to a broad general description.
In line with the overarching goal of reducing TAVR complications, the Navitor™ THV (Abbott Structural Heart, St Paul, MN, USA), has emerged as the latest iteration of the PORTICO™ platform (Abbott Structural Heart, St Paul, MN, USA), which is characterized by intra-annular leaflets.1,2 The Navitor™ valve’s design incorporates a balance of cutting-edge features to avoid multiple aspects of TAVR complications. Retaining the convenience of facilitating future coronary access, the Navitor™ valve stands out due to the strategic integration of an outer fabric cuff that actively mitigates the risk of PVL. In the opinion of the authors, the advancement in the delivery system has rendered the valve more deliverable due to improved system flexibility.
In this review, we provide an overview of the Navitor™ system and showcase the most recent clinical impact.
First-generation Portico™ valve: Design and clinical outcomes
The Portico™ THV system (Abbott Structural Heart, St. Paul, MN, USA) is a self-expanding THV featuring large open cells and intra-annular pericardial trileaflet bovine tissue.3 The valve is mounted in a self-expanding nitinol frame, with the ability to recapture, reposition and redeploy. The considerable open cellular area leads to an elevated ratio of tissue to frame at the annulus, thus reducing the risk of paravalvular leakage by enabling the valve tissue to adapt to the shape of calcifications present at the annulus. Available sizes include 23, 25, 27 and 29 mm.3 The valve obtained CE mark in 2012, 2013 and 2015 for the 23 mm, 25 mm, and both 27 and 29 mm valves, respectively.
In its conceptualization, the Portico™ THV was engineered to harness the superior attributes of other self-expanding valve platforms, for example the Medtronic Evolut™ valve, such as the ability to recapture, reposition and redeploy, along with superior haemodynamic performance. Additionally, it aimed to incorporate the benefits associated with contemporary balloon-expandable valve platforms, like facilitated coronary access due to its intra-annular leaflets and large open cells. Furthermore, the valve was designed with a pliable delivery system, enabling it to adeptly navigate intricate anatomical structures.
Several non-randomized studies in patients with high surgical risk have demonstrated safety and effectiveness of the Portico™ THV that align with those observed for other commercially available THV platforms, such as the Medtronic Evolut™ valve.4–6 In the IDE clinical study, the Portico™ system did not establish non-inferiority when directly compared with commercially available devices (supra-annular SEV or intra-annular BEV) in patients with high and prohibitive surgical risk.7 The primary safety endpoint included the composite of all-cause mortality, disabling stroke, life-threatening bleeding requiring transfusion, acute kidney injury requiring dialysis, or major vascular complication at 30 days. Notably, the drawbacks of the Portico™ system were higher rates of PVL (7.6% versus 1.3%, p=0.006), major vascular complications (10.7% versus 4.4%, p=0.02), rates of PPI (27.7% versus 11.6%, p<0.001), and 30 day mortality.7 The primary efficacy endpoint at 1 year, which included the composite of all-cause mortality or disabling stroke, was non-inferior between the groups (14.8% versus 13.4%, p=0.006).7
Navitor™ system: Second-generation Portico™ valve
The Navitor™ THV is the latest iteration and successor of the Portico™ valve and has the following key refinements compared with its predecessor (Figure 1).1,2
-
Large cell design to minimize coronary obstruction and improve coronary access, with curved aortic cells to reduce injury to native structures.
-
Dedicated inner and outer NaviSeal™ fabric cuffs, that actively synchronizes to the cardiac cycle in order to fill the calcification related gaps between the THV and virtual basal ring, as well as a landing zone without cutouts to improve sealing and reduce PVL.
-
Induction of a 14 Fr low-profile FlexNav™ delivery system, with improved deliverability and feasibility in patients with small peripheral access (up to 5 mm) and lower vascular complication rates.
Figure 1: Evolution of device technology from the Portico™ to the Navitor™ valve
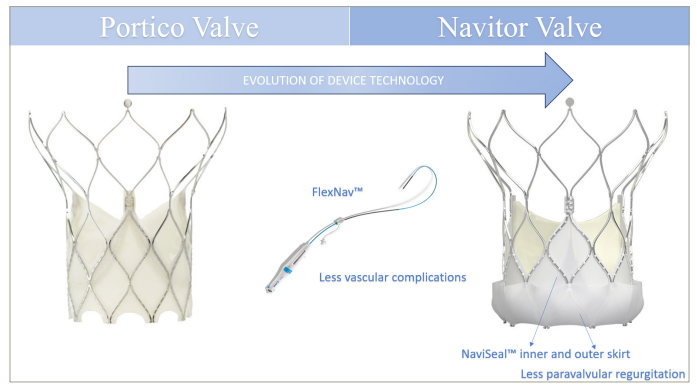
Figure was created and provided by Abbott for use in this paper.
With such advances, the primary benefits would be lower risk of PVL and vascular complications. The succeeding iteration of FlexNav™ delivery system (DS) from Abbott enhances the capabilities of the current Portico™ platform. It retains the original system’s flexibility, allowing for valve resheathing and repositioning before complete deployment. However, it goes further by enhancing deployment precision and offering one of the most minimal insertion profiles among all TAVR systems in the market, with the potential to access vessels as small as 5.0 mm. The 23- and 25 mm valve require a 14 Fr system compared with the larger size valves (27-, 29 mm) which require a 15 Fr system (at least 5.5 mm size vessel). The Navitor™ gained CE mark in 2020 and was approved in the USA in January 2023.8,9
The Portico™ NG is a prospective, multicentre study in 26 sites in the USA, Europe and Australia, included 260 patients with severe aortic stenosis (AS) who underwent Navitor™ THV implantation between 2019 and 2022 with a plan for a 5-year total follow-up.10 Most recently, the 30-day outcomes were published.10 The primary endpoint was all-cause mortality at 30 days, and the primary effectiveness endpoint was the occurrence of at least moderate PVL at 30-day follow-up. Other secondary endpoints included all-cause mortality, disabling stroke, life-threatening bleeding and major vascular complications, and advanced acute kidney injury at 30 days. The average age of patients was 83.4 years, with 57.3% being female, and 55.0% classified as NYHA (New York Heart Association) functional class III or IV. The mean STS-PROM (Society of Thoracic Surgeons-Predicted Risk of Mortality) score was 3.9%. Transfemoral access was achieved in 99.6% of the cases. The in-line sheath was used in 89.2% of patients. The majority of the patients (95.4%) underwent pre-balloon dilatation, while post-balloon dilatation occurence was 28.1%. Mean implant depth was 4.2 mm. The valve was successfully implanted in 97.3%. At 30 days, 1.9% of patients had died, 3.8% had severe life-threatening bleeding, 1.9% had a disabling stroke, 0.8% had stage 3 acute kidney injury, necessitating dialysis, and 4.2% had significant vascular complications. At 30 days, there were no patients with moderate or severe PVL. Of note, 0.8% (two patients) underwent PVL closure at the time of the procedure for moderate PVL. The PVL occurred in the setting of deep implantation of the valve and did not resolve with post dilation. Improvement in NYHA score was noted in 86.5% of patients.10
Comparison of the first-generation Portico™ and the Navitor™ systems
To date, there is limited data comparing the first-generation Portico™ to the Navito™ system. In the Portico NG study, the rate of 30-day PVL with the Navitor™ system was 0%, with significant improvement from prior reports with the Portico™ system (6.3% at 30 days in the PORTICO IDE study and 3.9% in the PORTICO I study).5,7,10 This is most likely related to the addition of the NaviSeal™ cuff to the valve design.
In regard to haemodynamics, both valves continue to have low post-implant mean aortic valve gradient and a large effective orifice area (EOA). PPI rate was 19% at 30 days in the Portico NG study, compared with 15% in the PORTICO IDE study.7,10 It is important to note that the cusp overlap technique was not universally used and this may have influenced the rate of PPI.
To our knowledge, there is only one published study comparing the two iterations of the valves. Eckel et al. aimed to compare the 30 day outcomes between the two THV systems in a retrospective study.11 A total of 139 patients with Portico™ and 137 patients with Navitor™ were assessed according to Valve Academic Research Consortium-3 (VARC-3) recommendations. Compared with Portico™, Navitor™ had significantly lower rates of more than mild PVL post-procedure (1.5% versus 7.2%, p=0.041). Additionally, severe bleeding rates (27.3% versus 13.1%, p=0.005) and major vascular complications (5.8% versus 0.7%, p=0.036) were lower in the Navitor™ group. Haemodynamic outcomes were comparable, and there was no difference in mortality between the two groups. The Navitor™ THV demonstrated improved in-hospital procedural outcomes, with favorable PVL rates and preserved haemodynamic outcomes. However, the incidence of PPI remained high in both groups (21. 6 % in patients with the Navitor™ and 15.3 % in those with the Portico™).11
Despite notable constraints within this study such as a modest sample size, the retrospective nature of the study, and an extended study period marked by considerable variations in technology and operator experience, the novel valve design of the Navitor™ has the potential to mitigate complications, including PVL, major bleeding and vascular complications. Durability data however is limited due to the recent release and implantation of this device. It remains to be seen how its durability will compare with other contemporary commercially available THV platforms.
Ongoing clinical trials
The Portico NG trial (Evaluation of the Portico™ NG (next generation) transcatheter aortic valve in high and extreme risk patients with symptomatic severe aortic stenosis; ClinicalTrials.gov identifier: NCT04011722) remains an ongoing study for patients with high and extreme surgical risk and severe symptomatic aortic stenosis, even after the publication of the 30 day outcomes.12 It will continue to monitor these subjects for 12 months, gathering data at various stages of their treatment, including screening, baseline, procedure, discharge, and follow-up assessments spanning up to 5 years. Furthermore, the study will also include a product size extension, using the larger size Navitor Titan™ valve, encompassing up to 90 subjects who will undergo evaluations similar to those in the Portico™ NG cohort.12 Similarly, the VISTA study (VISTA study [Navitor post-market clinical follow up study]; ClinicalTrials.gov identifier: NCT06008080) is assessing the safety and durability of the Navitor™ system in a global real-world setting.13
The VANTAGE clinical study (VANTAGE clinical trial evaluation of TAVR using the NAVITOR valve in a global investigation; ClinicalTrials.gov identifier: NCT04788888) aims to assess the safety and efficacy of the Navitor™ valve in individuals suffering from severe, symptomatic aortic stenosis, who present an intermediate or low risk of surgical mortality outside of the USA.14 Additionally, this trial will examine the safety and effectiveness of using the Navitor™ valve in a valve-in-valve scenario.14 The ENVISION clinical study (Evaluation of the Navitor transcatheter heart valve in low and intermediate risk patients who have severe, symptomatic, aortic stenosis requiring aortic valve replacement; ClinicalTrials.gov identifier: NCT05932615) will compare Navitor™ to a commercially available TAVR valve in patients with low or intermediate risk in the USA, and is expected to start in the first quarter of 2024.15
The Comfort study (Coronary re-engagement after random Navitor alignment [COMFORT STUDY]; ClinicalTrials.gov identifier: NCT05779787) explores the ability of coronary re-access with random Navitor™ valve implantation (without alignment) compared with the coronary alignment implantation technique.16 The study aims to assess if this simpler approach is non-inferior, addressing the prevalence of coronary artery disease and the increasing use of TAVI in younger patients. Findings may expand valve options for patients needing percutaneous coronary interventions post-TAVR, enhancing tailored treatment. Ultimately, it aims to determine predictors of difficult re-engagement, regardless of implantation technique, for a more personalized approach.
Comparison to other TAVR valves
There are three TAVR valve systems currently approved in the USA for commercial use. The SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA) is a balloon-expanded valve with a cobalt chromium frame and bovine pericardial valve which is approved for all risk levels. The Evolut™ supra-annular self-expanding valve (Medtronic CoreValve LLC, Santa Ana, CA, USA) which has a nitinol frame and porcine pericardial value which is approved for all risk levels. Finally, there is the Navitor™ valve by Abbott, which is an intra-annular self-expanding valve approved for extreme and high-risk patients. Both self-expanding valves have consistently shown better haemodynamic performance than the balloon-expanded valve.17 Pacemaker rates have tended to be higher in the self-expanding valves than in balloon-expanded valves. It should be noted however that improved haemodynamic performance is a function of the valve design, while pacemaker rates are more related to the technique of implantation. Pacemaker rates in the Evolut™ valve were in the double digits during the IDE trials. With the advent of the cusp overlap deployment techniques, pacemaker rates have fallen to 8.5% in the third quarter of 2023 (ACC 2021), secondary to more accurate placement of the annular level. It is reasonable to believe that the use of the cusp overlap technique, which was not used consistently in the Navitor™ trial, will decrease the Navitor™ pacemaker rates also. Currently however we are lacking head-to-head trials between these valves.
Conclusion
The Navitor™ valve, a second-generation TAVR device, has demonstrated notable improvements over its predecessor, reducing complications such as paravalvular leaks (PVL) and vascular issues. Comparative studies show a significant drop in 30-day PVL rates from 6.3% to 0%. Pacemaker rate remains high at 21.6%, but should be expected to decrease as the cusp overlap technique is adopted for this valve. While durability data is limited due to its recent introduction, the Navitor™ valve represents a promising advancement in TAVR technology, enhancing patient outcomes and minimizing risks.