The prevalence of aortic stenosis (AS) has grown over the last few decades, as life expectancy has increased. AS is the most common valvular disease in the developed world and is found in up to 9.8% of individuals aged ≥75.1–3 The wide range of ages, and the wide variability in the general health status in the older population, make patients with AS a very heterogeneous group. This population includes individuals who are fit and healthy, as well as frail individuals affected by multiple comorbidities, cognitive impairment and reduced mobility.
Balloon aortic valvuloplasty (BAV) is an endovascular procedure for stretching the stenotic aortic valve. The procedure consists in positioning and inflating a balloon in the aortic valve with the objective of increasing valvular area and releasing gradient. The gradient release is immediate, as well as the clinical improvement. BAV was first described by Cribier et al. as a simple, relatively safe and effective strategy for patients with AS.4 After the initial enthusiasm for this procedure,5 BAV was shown to have a high rate of symptom recurrence and restenosis at 6 months and a low impact on mortality.6 Consequently, the use of BAV reduced, and it was indicated only in patients with AS in cardiogenic shock or in special clinical situations as a bridge to aortic valve replacement (AVR), which was the standard of care in the 1990s.7 In 2002, Cribier et al. introduced transcatheter aortic valve implantation (TAVI), dramatically changing the landscape of AS treatment.8 After demonstrating the superiority of TAVI to optimal medical therapy in treating inoperable patients,9,10 TAVI needed to demontrate better outcomes in high-risk patients compared with AVR.11 Recently, TAVI was shown to be non-inferior to AVR in treating medium- and low-risk patients with AS;12–14 consequently, the number of TAVI procedures increased because it was a less invasive alternative. The tremendous success of TAVI as a minimally invasive and low-risk procedure, allowed us to provide a definitive treatment to patients ruled out for AVR. The interest in BAV was renewed as a bridging therapy to TAVI, since a relatively simple tool for improving the clinical condition of critical or frail patients was often required. The BAV increase mirrors that in TAVI;15 consequently, the number of BAV interventions has increased dramatically in the last few years.16
However, as the outcomes of TAVI continue to improve, the role of BAV will be defined according to its associated risk. The key to keeping this procedure viable is to demonstrate a low rate of complications, especially related to vascular access. This review aims to analyse the role of BAV in this evolving TAVI era. We will define indications, analyse results and describe complications. Finally, we will focus on technical improvements for reducing the risk of complications.
Balloon aortic valvuloplasty indications
BAV indications can be classified by considering the intervention as a bridge to AVR/TAVI or as destination therapy, whereby no further procedures are undertaken, other than repeated BAV.17 Nevertheless, there is a broad range of indications whereby BAV cannot be considered either as definitive or as a bridge to TAVI/AVR. These include diagnosing the impact of AS in a clinical situation, improving frailty, reducing the risk of non-cardiac interventions by releasing the aortic gradient and reducing the risk of waiting for definitive treatment. BAV is also indicated as part of the TAVI procedure for facilitating valve deployment and optimizing results, and can be used for treating intraprosthetic or paravalvular leaks. However, these former two indications will not be analysed in this review.
Balloon aortic valvuloplasty as a bridge to transcatheter aortic valve implantation or aortic valve replacement
Due to the high rate of restenosis, which is reported to be as high as 70% at 6-months follow up, BAV is considered a palliative procedure in most AS cases.6,18,19 The principal indication of BAV is as a bridge to a definitive treatment (i.e. TAVI or AVR). The concept of being a bridge to another procedure is used to improve the clinical condition of a patient at high risk of TAVI or AVR, enabling them to undergo the procedure.
Cardiogenic shock
Cardiogenic shock related to decompensated AS is associated with a poor prognosis.20 An emergency BAV allows immediate gradient reduction and cardiac output improvement, an indication that has been supported by current guidelines.21–24 Emergency AVR carries a prohibitively high risk in such patients, and therefore is not a viable option. More recently, TAVI was proposed as a valid alternative to AVR in emergency settings, since it has better outcomes with regard to haemodynamics, and does not require sequential interventions.23,25 However, BAV has undeniable advantages over TAVI in emergency settings because of its immediate availability, simplicity and low resource requirement, which makes it suitable in a broad spectrum of centres (i.e. low resources and high resources centres, medium and high complexity centres). BAV provides relief given by gradient reduction; consequently, this procedure should be offered as early as possible after clinical decompensation, since the time from inotropic support to BAV is strongly related to mortality.26 Therefore, whether BAV is immediately available can be decisive for determining in-hospital prognosis.26
Using BAV as a bridge to TAVI is critical for improving outcomes in AS-related shock.20 Indeed, this strategy has led to a decrease in the in-hospital and one-year mortality of this population over time.20 This improvement in prognosis, however, is mainly driven by the possibility of a definitive treatment after the BAV20,25,26 and can be achieved in up to 75% of patients with initial contraindication to TAVI/AVR.27
Severe aortic stenosis and non-cardiac surgery
BAV is a useful tool as a bridge to non-cardiac surgery, as it improves haemodynamic parameters and lowers non-cardiac perioperative risk.24 This indication for BAV should take into account several factors, including the urgency of non-cardiac surgery, the presence of symptoms, ventricular function28 and the impact of the clinical condition for which non-cardiac surgery is needed on the risk of definitive therapy for aortic stenosis (i.e. TAVI/AVR).
In pregnant patients with symptomatic severe AS, BAV improves haemodynamic outcomes and allows a safer delivery, usually by caesarean section, despite the medical therapy.29 The indications of BAV are not so clear for asymptomatic patients.29 TAVI could be a promising alternative in these cases; however, a longer procedure with more iodine contrast use is expected, and antiplatelet/anticoagulation combination therapy must be provided.
Expected prolonged waiting time for transcatheter aortic valve implantation
Given the high mortality associated with symptomatic severe AS and the potential for death amongst patients awaiting a TAVI procedure,30 the length of the waiting list is clearly a matter of concern. An elegant study by Wijeysundera et al. based on data from the PARTNER trials suggested that deaths related to TAVI wait time can be as high as 22.4% with a 180-day wait.31 Furthermore, they found TAVI-related mortality exceeded that of AVR when the wait time is longer than 60 days. Even though it is a controversial indication, BAV should be considered when a prolonged TAVI wait time is expected. Indeed, we can consider BAV, to improve prognosis if the TAVI wait time exceeds 30 days.
Balloon aortic valvuloplasty as destination therapy
Whether BAV may be effective as destination therapy depends on the immediate symptomatic relief gained. However, this is counterbalanced by the high restenosis rate associated with BAV, which was reported to be as high as 83% in early reports,32 but was found to be lower in more recent publications.33,34
With BAV as destination therapy, symptomatic relief is immediate and can be sustained for 1–2 years.35 One-year mortality was estimated to be about 40%.19,33,36 However, it was reported to be lower than 30% in a case series analysing the significance of gender in outcomes.34
BAV as destination therapy is indicated for patients who are not suitable for AVR/TAVI due to technical aspects or short life expectancy. Indeed, despite technical advances, up to half of patients referred to a dedicated TAVI centre did not receive the procedure because it was considered inappropriate for non-cardiac comorbidity.37 In this context, BAV is the only tool available for improving the patient’s clinical condition. Consequently, BAV presents as a relatively simple and low-cost procedure that can improve quality of life in patients with AS by easing their symptoms and reducing hospitalization. Furthermore, the simplicity of BAV allows us to adopt a strategy of repeated procedures with acceptable safety and potential outcome benefit. The survival rates of patients undergoing repeated BAV at 1, 2 and 3 years (84.2%, 58.7% and 42.8%, respectively) were found to be higher than those reported for untreated patients with AS.38
Even though BAV is considered a palliative procedure,6,18,19 clinical improvement is undeniable and there are observational data showing lower mortality with BAV at 1, 2 and 3 years compared with no invasive treatment.33,34,38,39
Other indications
BAV can be indicated as a bridge to decision-making in a broad spectrum of patients.
Balloon aortic valvuloplasty as a “diagnostic procedure”
BAV has been suggested as a “diagnostic procedure” for testing the potential benefits of a subsequent TAVI or AVR.20 Indeed, the impact of AS on ventricular function, the functional component of mitral regurgitation or pulmonary hypertension can be accurately established based on the response to gradient relief. From a clinical perspective, if BAV does not produce a good outcome in terms of AS symptoms or haemodynamic status, this can indicate whether a subsequent, more complex procedure would be beneficial. Finally, in low-gradient AS, BAV is the simplest way of improving the aortic valve area (AVA) and thus defines TAVI/AVR according to ventricular function improvement or symptom relief. The success of this approach varies and has been shown to allow definitive treatment in >75% of patients.16,27,40
Balloon aortic valvuloplasty in frail patients for defining strategies according to clinical response to gradient relief
Frailty is a clinical state of increased vulnerability related to an ageing-associated decline in reserve and function across multiple physiologic systems.41 It plays a pivotal role in defining the chance of recovering after TAVI/AVR, since even a successful procedure will not improve short- or medium-term quality of life or mortality in frail patients.42 Indeed, measures of disability or frailty, such as wheelchair dependence and low serum albumin, were established as preoperative predictors of poor outcomes after a successful TAVI.43 In this setting, BAV is an efficient triage tool for avoiding a futile TAVI procedure.
Moreover, BAV can improve the frailty status and lead to a TAVI procedure with better outcomes. Frail patients whose essential frailty toolset score improved after BAV achieved comparable prognosis to non-frail patients.44
Balloon aortic valvuloplasty for improving the clinical condition and defining prognosis
Older patients with severe AS have complex medical histories and non-cardiac comorbidities that require prompt intervention. If non-cardiac surgery is required, BAV improves clinical status and reduces the risk awaiting definitive treatment. Defining the strategy in these patients requires not only analysis of the clinical condition, but also the demographic situation and psychosocial support. BAV can be used as a comprehensive patient-assessment tool, thus defining whether a subsequent TAVI/AVR would be appropriate. In a recent Australian retrospective study, fewer than half of patients from a tertiary care centre who had undergone bridge-to-decision BAV were deemed suitable to proceed to TAVI/AVR after 12 months.16
Results and complications of balloon aortic valvuloplasty
Results
BAV is considered successful when the mean transaortic gradient is reduced by ≥50%. However, a successful BAV can have a broad spectrum of definitions, as relative gradient decrease (30 to 50%) or absolute gradient decrease (20 to 40 mmHg). Today, the rate of success regarding gradient and AVA improvement is >95% because a variety of excellent performance wires, catheters and balloons are available, and the procedure itself is not technically demanding.
Typically, transaortic gradient reduction and AVA increase the chances of immediate clinical improvement.
The short- and mid-term outcome is good, with expected clinical improvement extending up to 6 to 12 months,16 although some reports describe symptomatic improvement extending up to 1.5–3 years.45 The reported restenosis rate is high, with a broad range of values.15,46 Restenosis was found to be as high as 83% at 9 months follow-up.15,46 The high restenosis rate led to consideration of the procedure as a palliative intervention.32 However, it was proposed that meticulous technique can achieve a low rate of restenosis and a more favourable impact on outcomes.33 The correlation between clinical outcome and mean gradient reduction is not clear,47 even though a better prognosis was reported with post-BAV AVA ≥1 cm2, or AVAi ≥0.6 cm2/m2.33
Considering the high restenosis rate, repeated BAV was proposed as a strategy for sustaining the benefit of gradient relief, with good results and a low rate of complications.38
A better long-term outcome of BAV is obtained when the procedure is applied as a bridge to TAVI or AVR. In this scenario, the caveat of BAV restenosis is solved with the definitive throughout treatment performed later, and the mortality drops dramatically.33,47,48
Complications
BAV acute and 30-day mortality has been reported to range from <1 to 14%, the lowest values in recent years.16,49,50 In patients with shock, in-hospital mortality was reported to be as high as 66% in the early days of BAV use (in the 1980s and 1990s), dropping to 25% in past several years.20 Since the procedure is mostly performed in patients that are not suitable for AVR or TAVI, complications and mortality are related to the clinical situation of the population analysed. Low left ventricular ejection fraction at baseline, cardiogenic shock, high comorbidity index, coronary disease, pulmonary hypertension, baseline renal failure, and peripheral vascular disease are powerful predictors of procedural and in-hospital mortality. In terms of complications, acute aortic regurgitation and haematocrit drop independently predict death.15,17,47,51
Complications can be classified as those associated with the valve intervention and those related to the vascular access. Complications associated with the valve intervention are infrequent and represent less than 3% of of the total complications reported, but are usually catastrophic. They include aortic rupture, coronary dissection, severe aortic regurgitation and embolic stroke.47,52,53 Vascular complications lead to longer hospital stays, often require blood transfusion and surgical repair, and are strongly related to in-hospital death.54
Initially, the rate of complications associated with BAV was extremely high. In 1991, the Mansfield Registry reported 20–25% of patients having major complications, with vascular injury in 7–9% of patients and intra-procedure death in 3%.49 Improvements in technique and materials has led to better outcomes. Today, BAV is a safer procedure than in the early years of its use, with less vascular complications (3–5%), bleeding (1–2%) and lower intra-procedure deaths (0.5–2%).16,55 However, the safety of BAV remains a concern, as it is usually performed in frail and elderly populations that are particularly prone to bleeding, and vascular complications related to age, comorbidities, severe vascular disease or acquired coagulopathy.56
Balloon aortic valvuloplasty caveats and the impact of technical elements
The immediate benefits that BAV provides regarding functional status and mortality are not sustained because of the high rate of restenosis. Therefore, BAV is considered a palliative therapy for most patients, and safety is essential in this type of procedure. Indeed, the initial enthusiasm for BAV vanished due to evidence of high restenosis,6 and especially because of the high rate of complications, which were mainly vascular.
In recent years, BAV has become a relatively safe procedure with a high rate of success.
The improvements are related to BAV strategy and material elements, which are detailed in the following.
-
The anticoagulation strategy changed. BAV is performed under low-dose heparin, and it was proposed to be performed without anticoagulation, with lower bleeding and ischaemic complications than under heparin.55 A reduction in bleeding with the use of bivalirudin compared with heparin was reported, but with similar rates of vascular complications and death.57
-
Manually shaped extra-stiff wires were replaced by pre-shaped wires that reduce the risk of left ventricular trauma.
-
Rapid ventricular pacing is widely used to reduce balloon swinging and the risk of ventricular perforation and valve injury. Ventricular pacing can be performed through the 0.035” stiff wire and the need for a femoral venous puncture is avoided. This strategy is particularly useful in cases with small hypertrophic left ventricle.
-
The early BAV balloons required 13–14 Fr sheath. Low-profile balloons are currently available and can be used within 8–9 Fr sheath, but they have low-rated burst pressure and are compliant. Hour-glass-shaped balloons were developed and can provide higher stability. New non-compliant, non-occlusive, and rapid inflation-deflation balloons may provide better outcomes and safety.
-
Ultrasound-guided femoral puncture is widely used. It allows a precise puncture of the common femoral artery and avoids calcium spots and femoral vein perforation. Consequently, overall complications and closure device failure are reduced.58
-
Vascular closure devices have become the standard of care for large-bore sheaths. Even with the availability of low-profile balloons, severe calcified valves require non-compliant balloons, and >10 Fr sheaths may be necessary. Vascular closure devices potentially reduce vascular complications and facilitate expedited patient mobilization.59
-
The most innovative and game-changing approach for BAV is transradial access (TRA). This was first reported in 2012 in a small case series.60,61 It is well known that TRA reduces vascular bleeding and vascular complications compared with femoral access, even when vascular closure devices are employed,62 and we must expect a higher benefit if large-bore access is required. Patients who are elderly and frail are prone to vascular complications;63 therefore, a favourable impact of TRA is expected in this population. In addition, patients who are obese and female are expected to benefit the most from BAV.16 These patients are also a population with a high risk of vascular complications,63,64 and consequently TRA can be particularly useful in them.
The place of transradial access in the balloon aortic valvuloplasty toolbox
BAV has traditionally been performed through femoral access because it requires 10–12 Fr introducer sheaths. The use of low-profile compliant balloons for BAV was introduced with the purpose of reducing vascular complications, since smaller introducer sheaths are required,65 allowing TRA BAV feasibility.
The Safety and Feasibility of Transradial Mini-invasive Balloon Aortic Valvuloplasty (SOFTLY) study proposed that TRA BAV with low-profile compliant balloons and left ventricular pacing through the stiff wire was safe and feasible.66 Later, the SOFTLY II study confirmed the safety and feasibility of TRA BAV, which was shown to improve quality of life and frailty status in patients who are frail,44 with no vascular complications or bleeding.
One would expect a reduction in complication rates with TRA BAV, although no randomized trials comparing this to traditional femoral BAV have been conducted to date.
TRA BAV, however, has some caveats. First, low-profile balloons require 8–9 Fr introducer sheaths, therefore TRA success is expected to be low. Indeed, the population that is expected to benefit the most from TRA BAV are patients who are elderly and female, and most of them are not suitable for 8 Fr TRA. Second, the use of larger sheaths can increase TRA complications such as radial spasm and injury, radial thrombosis, radial artery avulsion and hand ischaemia. Third, the maximum balloon size available for 8 Fr sheath is 20 mm and some patients require larger balloons to achieve a successful BAV. Fourth, semi-compliant balloons required for TRA BAV have less predictable inflation diameters and can be less effective than non-compliant balloons, especially in severe calcified AS.
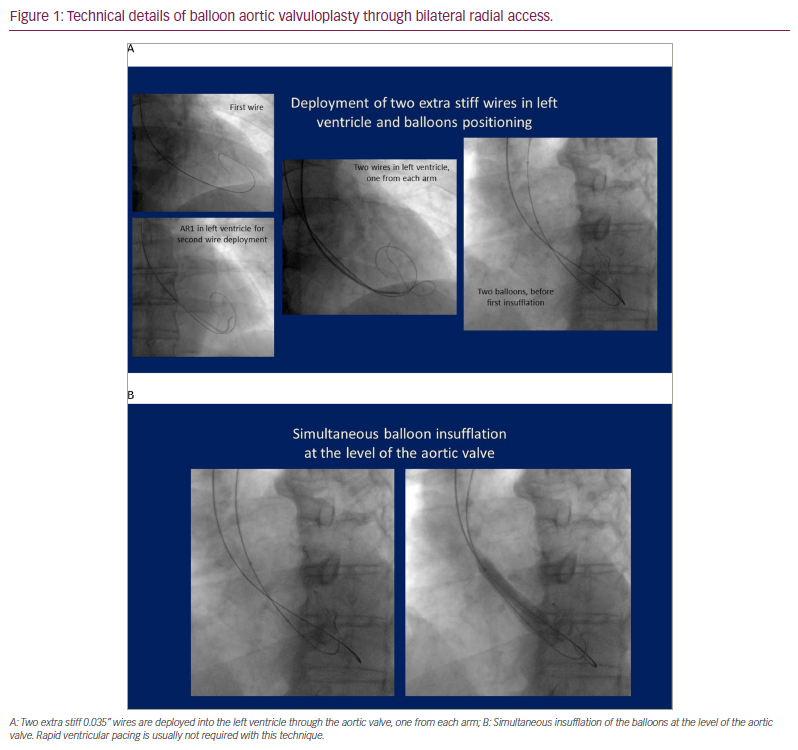
The double-balloon technique can sustain the advantages of TRA BAV, while reducing limitations and complications. Up to 10 mm and up to 12 mm non-compliant peripheral balloons can be delivered ”bare on the wire” through 6 Fr and 7 Fr sheaths, respectively. After achieving bilateral TRA (or ulnar TRA), two wires are positioned into the left ventricle and the BAV is performed by simultaneous insufflation of two non-compliant peripheral balloons (Figure 1). Cardiac pacing is not required most of the time because the system is stabilized, with a gentle insufflation of one of the balloons before the standard simultaneous insufflation of both balloons. Our group has used this technique in 14 selected cases since 2014, with good results. Although our work remains unreported, the technique itself has been described in three recent articles.67–69 The expected reduction of vascular complications obtained by performing bilateral TRA makes this approach a great alternative in selected cases, but its superiority over the traditional femoral approach has not been demonstrated. Indeed, it can potentially increase the risk of stroke and it is not clear if the simultaneous insufflation of two non-compliant balloons can lead to a higher rate of complications associated with the valve intervention.
Conclusions
BAV has undeniable value. From a logistical point of view, it is a relatively simple and affordable procedure that can be performed in non-TAVI centres. From a clinical perspective, BAV results in immediate relief of gradient and clinical improvement, with minimal surgical insult and minimal iodine contrast use. The current technology allows BAV to be performed with high rate of success, low procedure-related mortality, and low rates of vascular complications. Besides, BAV has no contraindications other than associated severe aortic insufficiency, severe coagulopathy or lack of vascular access.
The principal caveat of BAV is restenosis. Despite technical refinements, repeated BAV, and even post-procedural external beam radiation therapy,70 the rate of restenosis remains high and the impact of the procedure on medium- and long-term mortality, compared with medical treatment, is minimal or negligible.71 The high rate of restenosis led to the consideration of BAV as a palliative procedure in most cases. The procedure can be considered ‘curative’ only in patients with a short life expectancy.
On the other hand, TAVI is constantly improving, and it can be considered a definitive treatment. TAVI will be available for a broad spectrum of patients as it can overcome anatomic limitations, and costs are expected to decrease. Currently, despite being safer than AVR, TAVI is not a risk-free procedure and vascular complications are still a concern. The rate of major vascular complications is approximately 4%, and they have relevant impact on short- and long-term outcomes, including mortality.72
The classic role of BAV is as rescue therapy in patients with critical cardiac failure, as well as a bridge to TAVI/AVR or as destination therapy in patients with a short life expectancy. The current role of BAV is broader and includes allowing non-cardiac surgery in critically ill patients, evaluation of the impact of gradient reduction in cases with unclear expected benefit of TAVI, improving frailty and limiting the impact of a long wait time for TAVI on mortality.
The role of BAV must be determined on a case-by-case basis, taking into consideration the available facilities within the relevant healthcare system. In each case, we must consider the clinical situation, and the impact of AS on the functional status and life expectancy of the patient, aortic valve replacement risk (either TAVI or AVR) and waiting time to definitive therapy. Finally, the impact of the cost of TAVI on the healthcare system is relevant in many countries and determining the appropriate indication is crucial for limiting the number of futile procedures.CONCLUSION
BAV is utilized as a definitive treatment in some patients, but mostly for allowing definitive therapy in patients with an ominous prognosis. As a low-cost and versatile procedure, the use of BAV is expected to continue to grow, particularly as technical improvements allow operators to perform the procedure less invasively and more safely. In this context, TRA BAV is a valuable addition to the AS treatment ‘toolbox’.