A significant proportion of patients with atrial fibrillation (AF) who have a high stroke risk are currently being treated with oral anticoagulation (OAC), including direct oral anticoagulants (DOACs) and vitamin K inhibitors (VKAs).1 It is well known that the increased risk of thromboembolic stroke in patients with AF is primarily due to left atrial appendage (LAA) thrombus formation and consequent embolization.2 About 30–40% of patients with AF and an indication for anticoagulation do not receive anticoagulation due to contraindication such as high bleeding risk, medication intolerance or patient preference.3 Numerous studies have shown that left atrial appendage occlusion (LAAO), by excluding the LAA from the systemic circulation, effectively decreases AF-related stroke risk.4–11 The WATCHMAN™ (Boston Scientific, Marlborough, MA, USA) device was approved for the treatment of LAAO by the United States Food and Drug Administration (FDA) in 202012 after two pivotal randomized controlled clinical trials, the PREVAIL trail (ClinicalTrials.gov identifier: NCT01182441) and the PROTECT AF trial (ClinicalTrials.gov identifier: NCT00129545).13–16 However, they recommended anticoagulation after the procedure. The current recommendations indicate the prescription of OAC (VKA or DOAC) plus aspirin for 45 days, followed by dual antiplatelet therapy (DAPT) with aspirin (81–325 mg) and clopidogrel (75 mg) for 6 months, and aspirin (81–325 mg) indefinitely after that.17 LAAO can, however, result in certain procedural complications, including air embolism with subsequent stroke, pericardial effusions and device-related thrombus (DRT). DRT after LAAO has been shown to be associated with increased stroke risk.18,19 Anticoagulation regimen after LAAO remains a controversial issue and a challenge as a significant proportion of patients are at high risk of bleeding or intolerant to anticoagulation.
Aim and objectives
This article aims to comprehensively review the current literature regarding peri- and post-procedural anticoagulation with LAAO devices. The objectives of the review are to understand the indications and contraindications of the procedure; antithrombotic therapy before, during and after implantation of the device in patients with and without contraindications to OAC; the complications of the procedure; DRT; and incomplete occlusion of the appendage. The article also reviews the limitations of the current data and gaps in the literature.
Peri-procedural anticoagulation strategies
Thrombus in the LAA is the primary source of cardioembolic phenomena in patients with AF. Ninety percent of atrial thrombus cases were shown to be in the LAA in patients with non-rheumatic AF, as shown by the review by Blackshear and Odell.4 Anticoagulation in AF is recommended based on the CHA2DS2-VASc score. However, some patients have contraindications to long-term anticoagulation, including thrombocytopaenia, recurrent bleeding from various sites (gastrointestinal, respiratory or genitourinary), prior severe bleeding, recurrent falls or medication non-compliance. Hence, the concept of closure of the LAA and of possibly removing the need for anticoagulation has been proposed and has been around since 1948.20 However, there is still controversy around the optimal post-interventional regimen of antithrombotic drugs. Endovascular devices for LAAO include the WATCHMAN, Amplatzer™ (Abbott, Chicago, IL, USA) and WaveCrest® (Johnson & Johnson, New Brunswick, NJ, USA) devices. The LARIAT system (SentreHeart Inc, Redwood City, CA, USA) is a percutaneous device used ‘off label’ in the USA.17 For catheter-based LAAO, a thrombus visualized either by a transoesophageal echocardiogram or coronary computed tomography angiography scan is considered a contraindication.21 Antithrombotic drugs such as unfractionated hepwarin guided by partial thromboplastin time or oral anticoagulants (e.g. DOACs or VKAs) for ≥4 weeks are recommended in such situations.21 LAAO is performed after the documented resolution of the thrombus.
Anticoagulation during the procedure
During device implantation, unfractionated heparin is commonly used among intravenous anticoagulation agents. In the PROTECT AF trial, an international normalized ratio of <2.0 was recommended.16 After the transseptal puncture, weight-adjusted heparin (70–100 IU/kg) was administered, and an activated clotting time of >200 s was maintained during the procedure, while aspirin (81–325 mg) was started at least a day before the procedure.16,22 However, some operators might be comfortable performing the procedure while the patient is on VKA (with INR>2.0) or on a DOAC.
Anticoagulation after the procedure
The PROTECT AF16 and PREVAIL13 studies were both conducted on patients with AF, who received warfarin plus low-dose aspirin for 45 days, followed by low-dose aspirin plus clopidogrel 75 mg (DAPT) for 6 months and by aspirin 325 mg daily lifelong after that. Both studies showed that the efficacy of LAAO was non-inferior to anticoagulation alone.
A meta-analysis of both trials confirmed these results.23 Bleeding complications occurred in about 1.2% of the patients on aspirin and OAC during evaluation 45 days after the implant.21,23 Subsequently, 0.6% of these patients had a bleeding event when they received DAPT in the following study period.24 In patients with contraindications to OAC, decisions regarding anticoagulation after the procedure and stroke prophylaxis can be particularly challenging.
Another remarkable study is the EWOLUTION trial, which reported outcomes at 1 and 2 years (ClinicalTrials.gov identifier: NCT01972282).25 In this trial, 72% of patients could not adapt to harsh anticoagulation and hence were switched to DAPT, single antiplatelet therapy or no medication. A total of 66% were changed to single antiplatelet or no therapy after 3–6 months; by the end of the study, 84% of the active patients were only on single antiplatelet therapy or none. Despite these switches, the outcomes of the WATCHMAN implant were favourable. Patients with the LAAO device had consistently low rates of non-procedural bleeding and stroke.
There are relatively fewer studies on surgical LAAO. In the LAAOS III trial (ClinicalTrials.gov identifier: NCT01561651), patients with AF were randomized to either undergo LAAO at the time of cardiac surgery for another indication or to not undergo the procedure and continue with anticoagulation.26 Stroke or systemic embolism occurred in 4.8% of participants in the occlusion group and 7.0% of participants in the control group; moreover, there was not a significant difference in the incidence of peri-operative bleeding, heart failure or death between groups. Another study investigated antithrombotic therapy after the LAAO procedure.27 It showed an association between adding aspirin to anticoagulation and an increased risk of bleeding; anticoagulation alone had a similar reduction in major adverse events but no increase in ischaemic events. Patients who were discharged on DAPT did not differ significantly in terms of risk of adverse events compared with aspirin and warfarin after adjustment of risk profiles.
The European Society of Cardiology guidelines from 2016 make a Class IIb recommendation for LAAO for stroke prevention in patients with AF who have contraindications for long-term anticoagulation.28 The 2020 update of the European Heart Rhythm Association (EHRA)/European Association of Percutaneous Cardiovascular Interventions (EAPCI) expert consensus statements assert that LAAO is acceptable in patients with contraindications to anticoagulation.21 Published in 2019, the American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Rhythm Society (HRS) focused update of 2014 guidelines also provided a Class IIb recommendation for percutaneous LAAO for patients with AF with increased risk of stroke and who have contraindications to long-term anticoagulation.11,29 This update also gave a Class IIb recommendation for surgical LAAO for patients with AF undergoing cardiac surgery.
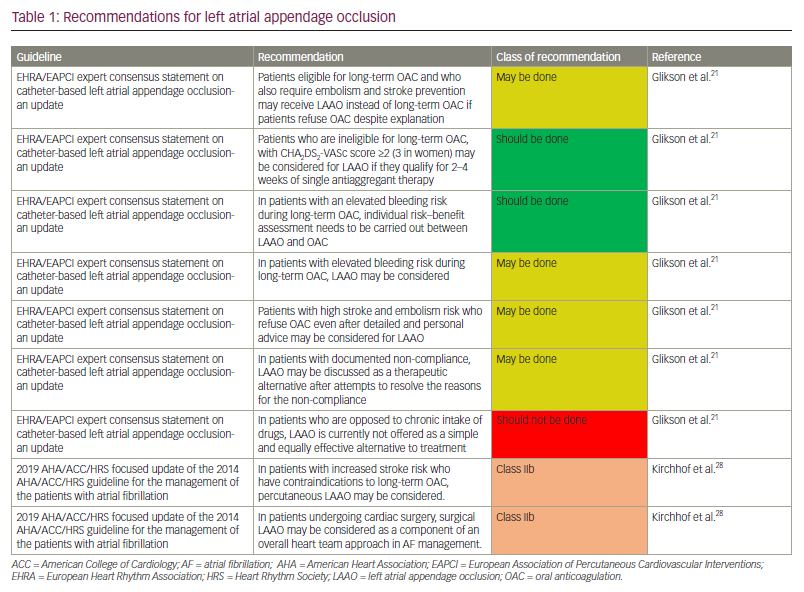
The recommendations are detailed as follows (Table 1). The EHRA/EAPCI consensus update provided five clinical situations and discussed indications for LAAO in each.21,30 They recommend that LAAO “may be done’’ instead of OAC in patients who are eligible for OAC and require stroke prevention if they refuse OAC. They also recommend LAAO in patients with AF with absolute contraindications for long-term OAC with a CHA2DS2-VASc score of ≥2 (3 in women) who can be given 2–4 weeks of single antiaggregant. The contraindications that are defined in the consensus update are the risk of major bleeding (especially disabling or life-threatening bleeding due to an untreatable source of intracranial/intraspinal bleeding or severe gastrointestinal, pulmonary, or urogenital source of bleeding that cannot be corrected) and severe side effects under VKA and/or contraindications to the novel OACs. A risk-benefit analysis needs to be performed between OAC and LAAO in patients with an elevated bleeding risk while on long-term OAC, and decisions should be made accordingly. They also give a weak recommendation to consider LAAO in the same clinical situation. In patients unwilling to take OAC even after extensive patient education, they weakly recommend considering LAAO and discussing it as a therapeutic alternative after maximum attempts to resolve the reasons/issues for non-compliance. The AHA/ACC/HRS 2019 guideline updates weakly recommend percutaneous LAAO in patients with AF at increased risk of stroke with contraindications to long-term OAC.11 They also weakly recommend surgical LAAO in patients with AF undergoing cardiac surgery, as a component of an overall heart team approach in managing AF.
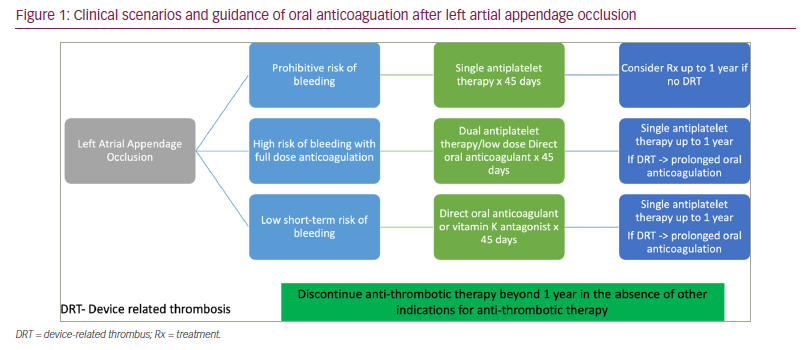
Based on the PROTECT-AF study, it is recommended to prescribe anticoagulation (DOAC or VKA) plus aspirin for 45 days, followed by DAPT (aspirin plus clopidogrel) for 6 months and aspirin daily thereafter.31 However, DAPT (aspirin and clopidogrel) for 6 months after the procedure is recommended for individuals with absolute contraindications to OAC, as shown in the ASAP study (ClinicalTrials.gov identifier: NCT00851578),32 or for a shorter period of 1–3 months for individuals who are at high risk of bleeding.33 As many patients with AF have concomitant atherosclerotic vascular disease, LAAO may allow us to use an ideal regimen for patients with coronary artery disease with DAPT or a low-dose DOAC and single antiplatelet therapy.34,35 The WOEST trial (ClinicalTrials.gov identifier: NCT00769938) observed excess mortality in patients with AF undergoing percutaneous cardiovascular intervention who were managed with triple therapy of DAPT and OAC, compared with those undergoing dual therapy of DOAC and clopidogrel.36
A summary of the clinical scenarios and guidance regarding OAC/antithrombotic therapies post-LAAO are provided in Figure 1.
Complications
The safety and complication numbers of the WATCHMAN device have improved significantly over time due to increased experience and improved equipment. A registry with more than 38,000 WATCHMAN implantations shows a procedural success rate of 98.3%.37 A study compared the outcomes between the PROTECT AF study and a non-randomized Continued Access Protocol registry of patients undergoing WATCHMAN implantations after the randomized trial and found a decline in procedure- or device-related safety in 7 days in the non-randomized registry compared with the randomized trial.38
Potential complications can arise from the procedure or due to the device. Procedure-related death was reported to occur in 0.19% of cases and cardiac arrest in 0.24% of cases by the National Cardiovascular Data LAAO Registry.37 Major vascular complications occur peri-procedurally relatively more often than other complications, accounting for 0.15% according to the registry; this may be due to vein puncture or transseptal puncture, for example. The incidence of pericardial effusion has also decreased over time due to increased operator experience, with the most recent evidence indicating a tamponade incidence rate of about 1.3%.39,40 Concerning peri-procedural stroke events, ischaemic strokes accounted for 0.12% of cases and haemorrhagic accounted for 0.01% of cases.37 Strokes can be caused by device-related leaks, DRT and air embolism. Device embolization occurred in 0.07% of the patients included in the registry.37 Device erosion is a rare but devastating complication.
DRT aetiology is likely multifactorial and differs between patients.41 It is more common in patients with reduced left ventricular systolic function, larger LAAs, and higher CHA2DS2-VASc scores.19 It can occur late following LAAO, and a large number of patients recur and have it after successful resolution.42,43 DRT is clinically significant because its association with thromboembolic events is well established. Several studies have shown an increased incidence of stroke, systemic embolism, and death among patients with DRT.19,42,44,45 Several risk factors have been proposed to predict DRT;44,46 however, emerging concepts in DRT also include the possible role of the flow dynamics on thrombus formation and early detection of DRT precursors using cardiac computed tomography.47 Treatment is usually 6 weeks of OAC, and the extension of the treatment depends on bleeding risk and other contributing factors such as persistent device leak or spontaneous contrast in the left atrium.48,49 However, DRT is challenging in clinical practice as there are still several management issues, including the fact that most patients are not suitable for prolonged or intensified anticoagulation, the persistence of DRT in 20–25% of patients despite anticoagulation treatment, high recurrence rates and uncertainty of the management of different DRTs, especially with highly mobile or/and large thrombi.43–45
Peridevice leak (PDL) incidence varies widely, as there is a lack of consensus on leak detection and classification methods.47 The cutoff of what is considered to be a PDL differs widely between studies. There are several limitations to studies assessing PDL effect on outcomes, such as the large sample size requirement (as the rates of stroke or systemic embolization post-LAAO are low), the variability in the definition of significant and nonsignificant leaks (e.g. >3 mm, >5 mm), the variation in the classification used in clinical practice, and the current treatment for large leaks being anticoagulation, which confounds the assessment of the impact of PDL.50,51 The management of PDL remains uncertain as the current literature is limited. In smaller PDLs, watchful waiting has been suggested.24,52,53 Closure of the PDLs has been studied and showed complete or near-complete obliteration with low complication rates; however, the long-term efficacy is currently unknown.51,54–57
Contraindications to anticoagulation
While there have been several observational studies58,59 targeting patients with a high thromboembolic risk but contraindications to OAC or systemic anticoagulation, no randomized data exist for such population. Nonetheless, the EHRA/EAPCI consensus statement indicates that LAAO is safe and effective despite the absence of even temporary OAC.21 DAPT is usually recommended for 1–6 months followed by lifelong antiplatelet therapy. Management usually involves 6 weeks of anticoagulation but can be extended depending on bleeding risk and other factors, such as PDL or spontaneous contrast in the left atrium.48,49
Limitations
The limitations of the study are largely due to the limitations of the trials described. The big trials, PROTECT AF and PREVAIL, had small sample sizes (707 and 407, respectively).13,16 Reviewers for the FDA found PROTECT AF’s handling of haemorrhagic strokes to be inconsistent.60 In addition, a second co-primary efficacy endpoint of systemic embolism or ischaemic stroke occurring more than 7 days following surgery were included in the PREVAIL trial.16 Two meta-analyses of PROTECT AF and PREVAIL showed a similar pattern of outcomes to the original studies, with greater rates of ischaemic stroke and lower rates of haemorrhagic stroke and cardiovascular/unexplained mortality.15,23 From the current studies, there are comparisons with VKAs but not with oral anticoagulants and LAAO.
Gaps in literature and pending trials
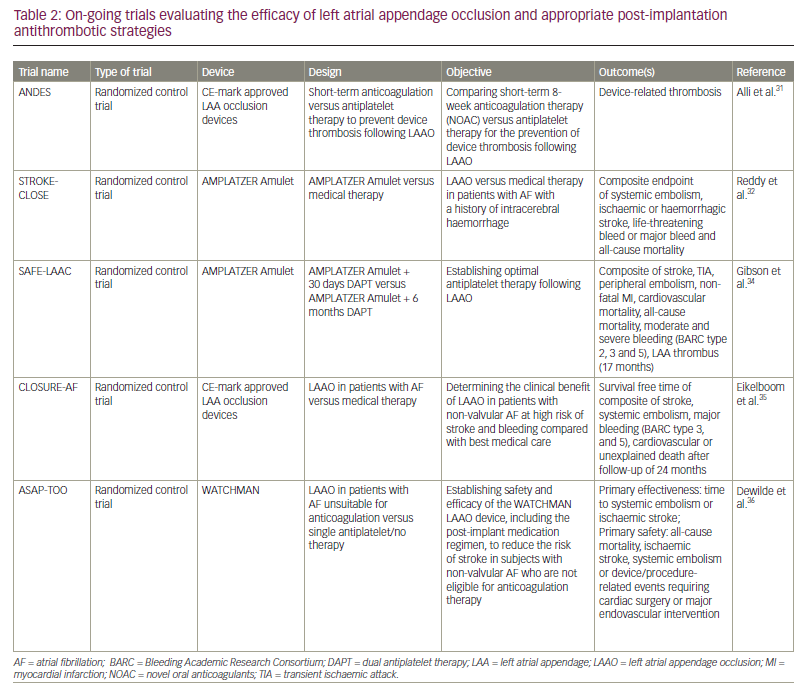
As previously mentioned, there are no trials with a large sample exploring the efficacy of LAAO; furthermore, no trials directly compare DAPT and OAC or the different devices. The ANDES trial (Short-term anticoagulation versus antiplatelet therapy for preventing device thrombosis following LAAO; ClinicalTrials.gov identifier: NCT03568890) will compare short-term novel OAC with antiplatelet therapy for patients after LAAO.61 The STROKECLOSE trial (Prevention of stroke by LAAO in AF patients after intracerebral hemorrhage; ClinicalTrials.gov identifier: NCT02830152) will compare LAAO with Amplatzer Amulet™ (Abbott, Chicago, IL, USA) or medical therapy in patients with previous intracerebral haemorrhage.62 The SAFE-LAAC trial (Optimal antiplatelet therapy following LAAO; ClinicalTrials.gov identifier: NCT03445949) will compare 30 days versus six months of DAPT after LAAO.63 The CLOSURE-AF trial (LAAO in patients with AF compared to medical therapy; ClinicalTrials.gov identifier: NCT03463317) will compare LAAO versus medical therapy in patients with a high risk of bleeding.64 The ASAP-TOO trial (Assessment of the WATCHMAN™ device in patients unsuitable for OAC; ClinicalTrials.gov identifier: NCT02928497) will compare patients with contraindications to anticoagulation after LAAO with WATCHMAN receiving single and DAPT.65 All on-going trials are listed in Table 2.
Conclusions
Percutaneous LAAO is increasingly being used for stroke prevention in patients with AF, especially in patients with high bleeding risk and with contraindications to anticoagulation. On-going trials will potentially help clarify the appropriate antithrombotic therapy post-LAAO and help reduce post-procedural complications.