Hypertrophic cardiomyopathy (HCM) is a genetic cardiovascular disease affecting 1 in 500 individuals worldwide.1 The disease pathogenesis of HCM is characterized by hypertrophy and myocyte disarray, along with muscle fibrosis on histopathology and intramural hyperplasia of the coronary arteries.2 HCM is diagnosed in patients with left ventricular hypertrophy having a maximum left ventricular wall thickness ≥15 mm in the absence of any other cardiac or systemic diseases that may explain the hypertrophy.3 Significant heterogeneity is present in the clinical presentation of this disease, particularly with respect to both onset of symptoms and intensity of symptoms at presentation.3 For example, some patients can present with mild dyspnoea, while others can present with arrhythmias, heart failure or sudden cardiac death.
More than 60 years have passed since the first modern description of the disease was published in 1958. Despite significant development in our knowledge of the disease’s pathophysiology and pathology, and the major progress made in surgical therapy and preventive measures, the arsenal of HCM medical therapy remains limited to beta adrenergic blockers, calcium channel blockers and disopyramide.4,5 Furthermore, these medications are not disease-specific to HCM and are in many cases inadequate, thus many patients require further septal reduction interventions for symptomatic reduction and survival improvement. Previous trials using other medical therapies like trimetazidine, ranolazine, eleclazine, spironolactone, perhexiline and losartan have unfortunately been unsuccessful.6–11
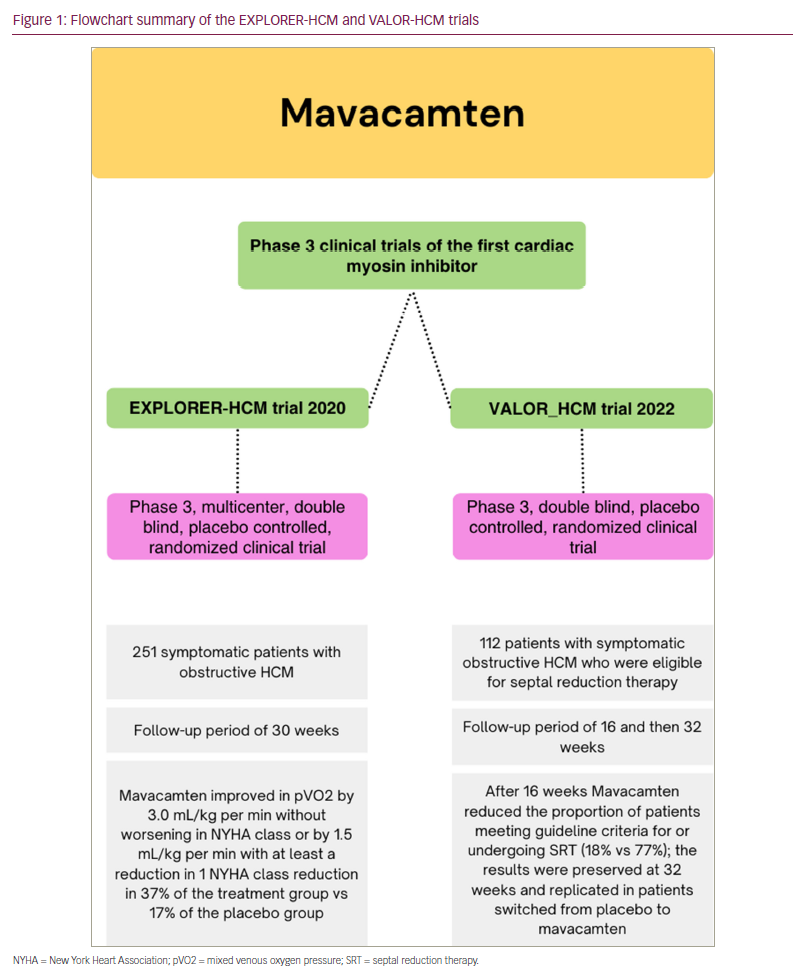
Mavacamten is a novel small molecule that inhibits cardiac myosin adesonine triphosphatase, causing a reduction in the hypercontractility and improvement in compliance by inhibiting the binding of myosin to actin, thus inhibiting the sarcomere force, an essential mechanism based on current knowledge of HCM disease pathology.12 To date, only two phase III randomized clinical trials have compared mavacamten with placebo in patients with HCM. These are the EXPLORER-HCM and the VALOR-HCM trials (Figure 1).13,14 Both trials reported positive outcomes and safety profiles.
The EXPLORER-HCM trial
The EXPLORER-HCM trial is a phase III double-blind randomized controlled clinical trial published in 2020. A total of 251 patients with symptomatic obstructive HCM were enrolled. This pivotal study has shown that treatment with mavacamten in patients with obstructive HCM leads to improvement in exercise capacity, New York Heart Association functional class, left ventricular outflow tract (LVOT) obstruction and health status.13 Currently, an extension study of the EXPLORER-HCM trial, the MAVA-LTE trial, is on-going (ClinicalTrials.gov Identifier: NCT03723655).15
The VALOR-HCM trial
In the recently published results of the VALOR-HCM trials, Desai et al. assessed the efficacy of mavacamten compared with placebo in 112 patients with symptomatic obstructive HCM who were on maximally tolerated medical therapy, and met the criteria to be referred for septal reduction therapy (SRT). The authors have shown a significant reduction of the number of patients still meeting the guideline criteria for SRT in the treatment group compared with placebo at 16 weeks (17.9% compared with 76.8% respectively, p<0.001).14 Moreover, at 32 weeks, the reduction in the proportion of patients who were SRT-guideline eligible was sustained in the macavamten group (10.7%). Additionally, a similar reduction was noted in patients originally placed in the placebo group and then crossed to mavacamten treatment (13.5%).16 Furthermore, the study has shown improvement in secondary outcomes, notably diastolic function grade, post-exercise peak LVOT gradient, New York Heart Association functional class and cardiac biomarkers (troponin and N-terminal pro B-type natriuretic peptide).
The side-effect profile of mavacamten
Both the EXPLORER-HCM and VALOR-HCM trials have reported that mavacamten was well tolerated among the study participants, who reported minimal side effects. The EXPLORER-HCM trial reported treatment-emergent adverse events as generally mild. VALOR-HCM reported three patients with serious adverse events; two patients with atrial fibrillation and one with coronavirus disease 2019.14 Being a well-tolerated therapuetic option is of great importance as these patients are spared from invasive SRT. Whether surgical septal myectomies or alcohol septal ablations, these procedures carry a significant risk of postoperative mortality and morbidity on top of a risk of procedure failure with the need to reoperate.17,18 Although these adverse events are less likely to occur in high-volume, experienced HCM centres, the scarcity of such centres worldwide means that the need for a non-invasive alternative is crucial.19 Being a myosin inhibitor, however, and thereby causing a negative ionotropic effect, a reduction in the ejection fraction is a potential side effect. Yet an ejection fraction of <50.0% was only reported in 6.0% and 3.6% in the EXPLORER-HCM and VALOR-HCM trials, respectively, all of which resolved after temporarily stopping the medication.
The limitations of mavacamten
A major point to consider is the commercial availability of mavacamten, with access sometimes dependent on the patient’s insurance. In the USA, its prescription and use are organized by the risk evaluation and mitigation strategy programme.20 This necessitates frequent echocardiography follow-up for dose titration based on the LVOT gradient and left ventricular ejection fraction, in order to identify patients who might have a significant reduction in the ejection fraction and develop heart failure.
Knowledge of the effectiveness of mavacamten is limited to a few studies that assessed its efficacy in symptomatic obstructive HCM, with the longest follow-up period reaching less than a year. More studies are to be publised in the next 5 years. Earlier detection of HCM is becoming feasible, due to novel technologies and increased awareness. However, with increased early detection, only a small proportion of the total number of individuals with HCM are represented in currently available studies. Knowing that mavacamten’s mechanism of action matches the disease pathophysiology of HCM and the successful outcomes it had in obstructive HCM, it is only natural to ask ourselves about the potential use of mavacamten both in patients with non-obstructive and asymptomatic HCM before obstructive symptoms occur. Currently, the ODYSSEY_HCM (ClinicalTrials.gov Identifier: NCT05582395) is an on-going phase III trial assessing mavacamten compared with placebo in 420 non-obstructive HCM patients.21 Moreover, one could even think about the use of mavacamten in cardiomyopathies of various aetiologies different to HCM, such as dilated cardiomyopathy and amyloid cardiomyopathy. Focus should be placed on conducting larger studies in future, to reproduce, validate and assess the long-term sustainability of the previous results, in order to provide incremental therapeutic value.
Conclusion
Mavacamten has been proven to be an effective and well-tolerated medical therapy in patients with obstructive HCM. In conclusion, with the development of novel disease-specific medical therapy, we have entered a new era in the treatment of HCM. Light should be shed on multidisciplinary approaches of precision medicine that implement our refined and advanced technologies for better patient outcomes.