In recent years, patients undergoing non-emergent percutaneous coronary intervention (PCI) procedures have become increasingly older, with a more significant comorbidity burden and complex coronary disease.1 Many of the patients who undergo elective or urgent PCI are surgical turndown patients, for whom PCI is the only revascularization option.2 For these high-risk patients, more interventionalists are opting for prophylactic mechanical circulatory support (MCS) – namely, percutaneous left ventricular assist devices (pLVADs), such as the Impella® heart pump (Abiomed, Danvers, MA, USA) – to prevent haemodynamic collapse during the PCI procedure.3
Access-related complication rates for high-risk PCI (HRPCI) performed with large-bore pLVAD support have varied significantly across published studies,4 with one large US healthcare analysis showing a 2.6-fold variation in bleeding events with Impella across hospitals.5 This reported variability in complication rates is likely related to multiple factors, including varying definitions of vascular and bleeding complications; furthermore, reports often do not identify whether bleeding events were access-related.4 Other factors include anticoagulation practices, use of optimal access strategies, use of closure devices and post-procedural care.5 While pLVADs for HRPCI provide the necessary support for certain high-risk patients to prevent haemodynamic collapse during PCI, they are associated with highly variable bleeding rates due to several factors, including differing definitions of major bleeding and differences between centres in access strategy, closure strategy, anticoagulation and post-procedural care.
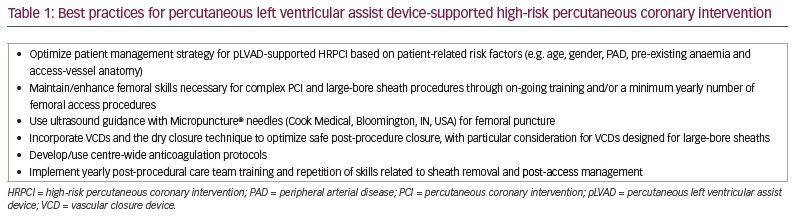
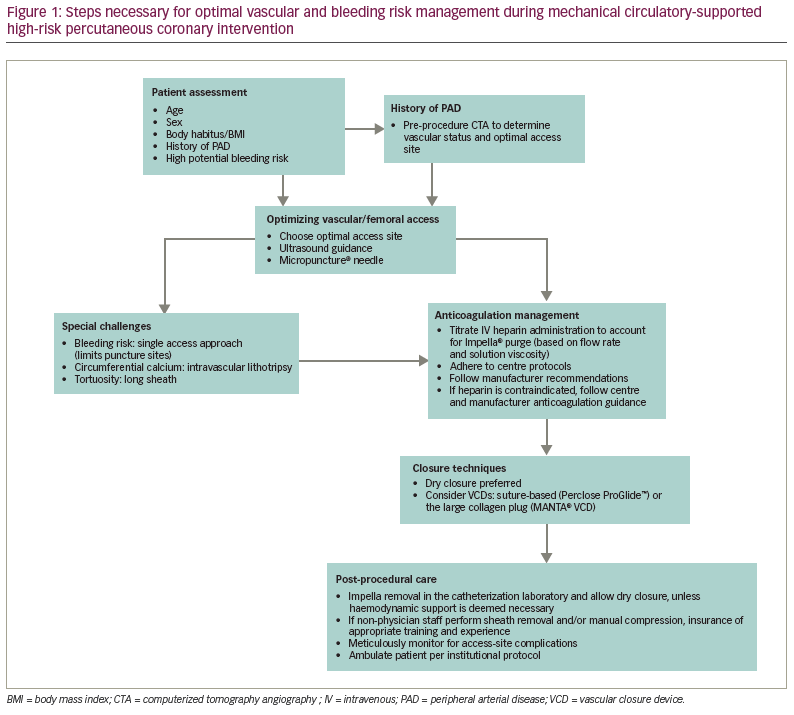
Our aim is to review the evidence and identify the best practices associated with each of these well-known factors of bleeding complications (summarized as access/closure, anticoagulation and post-procedural care) so that, ultimately, more practitioners may adopt these best practices and variability in bleeding complications – as well as excess bleeding complications – is reduced in the future (Table 1, Figure 1).
Identification and management of patient-related risk factors
Age and vascular disease
Age and peripheral arterial disease (PAD) are two important risk factors for vascular and bleeding complications during PCI.6 Older patients are potentially at increased bleeding risk due to diminished drug metabolism and changes in vascular and tissue integrity.7,8 An analysis of elective PCI outcomes found that patients aged >80 had a nearly identical rate of in-hospital major adverse cardiac and cerebrovascular events compared with younger persons (2.9% versus 2.8%); however, bleeding occurred at a significantly higher rate (3.3% versus 0.3%; p<0.0001).7 Another study found that older age was significantly associated with ecchymosis post PCI (p=0.004) and also identified lower body surface area as a significant factor for haematoma formation, with each 0.1 increase in body surface area associated with a 0.88x risk for developing a haematoma.8 In the PROTECT II trial, rates of bleeding and vascular complications were low and similar regardless of age (≥80 or <80) for both intra-aortic balloon pump and patients with pLVAD; however, this was a highly selected patient population, as it excluded patients with severe PAD.9 Thus, older patients, particularly those with PAD, require careful vascular access management and bleeding risk strategies.
Hypertension
Patients with arterial hypertension are at increased risk for both access- and non-access-related bleeding events during PCI. A large retrospective analysis of the Intracoronary Stenting and Antithrombotic Registry randomized controlled trial populations found that patients with a history of hypertension had a 41% greater risk for bleeding events, in particular for non-access-site bleeding (p=0.002), whereas systolic blood pressure at the time of PCI increased the risk for access site bleeding (p=0.018).10 Patients with hypertension had similar one-year mortality compared with normotensive patients; however, mortality was significantly higher in hypertensive patients with a bleeding event. Thus, optimizing blood pressure control prior to elective, supported PCI is important.
Female gender
Several studies have identified female gender as a significant risk factor for PCI access-site or bleeding complications.11–13 This risk is partly due to women having smaller peripheral artery luminal diameters than men regardless of body mass index (BMI).14 The sex hormone levels in women may also play a role in the level of coagulation factors, as well as having, on average, a reduced muscle mass, as muscles provide a degree of intrinsic compression.11,15
In a large catheter-based ventricular assist devices registry analysis of pLVAD-supported HRPCI, the rate of vascular complications was statistically similar across genders; however, 9.58% of women had bleeding that required transfusion compared with 5.30% of men (p=0.019).16 Baseline haemoglobin levels were significantly lower in women (average 11.0 g/dL compared with 13.1 g/dL in men); after adjusting for baseline anaemia, no significant difference in bleeding events was found.
Patients with low baseline haemoglobin levels and/or BMI (characteristics more often observed in women) are particularly at risk for bleeding complications during HRPCI. A pooled analysis of the OASIS 5 and 6 trials of patients with acute coronary syndromes undergoing PCI identified an association between low baseline haemoglobin levels and overall, procedure-related, and non-procedure related bleeding at 30 days, with the authors observing that risk decreased by 6% for every 1.0 g/dL increase in haemoglobin above 10.0 g/dL through to 15.9 g/dL.17
A large-scale Japanese registry analysis of 10,142 patients undergoing PCI found that patients who were underweight had higher overall complication rates, mortality, propensity for cardiogenic shock and bleeding complications. Bleeding complications were reported in 10% of patients who were underweight compared with rates of <5% in patients who were normal weight, overweight and obese.18 While necessary for all patients, women require thoughtful decisions about choice of access site, systemic risk management, and meticulous access and closure techniques.
Complex, interrelated risk factors for percutaneous coronary intervention
In the PCI bleeding risk model developed by Rao et al. using the CathPCI Registry, bleeding rates were found to vary significantly depending on a patient’s bleeding risk score, derived from a risk tool comprising the following 10 variables: BMI, age, gender, baseline haemoglobin, PCI status (urgent or elective), ST-segment elevation myocardial infarction, cardiac arrest within 24 hours of PCI, shock, previous PCI and chronic kidney disease.19 Risk of PCI bleeding was estimated to range from 0.9% (for patients assigned a bleeding risk score of 0) to 86.0% (for patients assigned the maximum risk score of 210), illustrating that multiple baseline factors play a significant and interrelated role in procedure-related bleeding risk. Notably, the overall PCI bleeding rate of 5.8% identified in the CathPCI Registry for all clinical circumstances is similar to the median bleeding rate identified in a recent review of bleeding risk with Impella-supported HRPCI (5.2%).4
Bleeding risk factors for transcatheter aortic valve replacement
A recent analysis by Gupta et al. found that the CathPCI bleeding model demonstrated a lower goodness-of-fit when PCI was accompanied by transcatheter aortic valve replacement (TAVR).20 Specifically, access-site bleeding (CathPCI Registry) was significantly higher in patients undergoing PCI with TAVR compared with PCI alone (19.7% versus 1.9%; p<0.001).20 Although this was a small, single-centre analysis, the results underscore the heightened bleeding risk associated with large-bore procedures, emphasizing the need for specific bleeding models to assess the critical factors in this population and facilitate preventive patient management.
Although there is a dearth of analyses assessing risk factors for bleeding in the setting of pLVAD-supported HRPCI, considerable research has been conducted assessing risk factors for periprocedural bleeding in TAVR. A recent National Inpatient Sample analysis of patients undergoing TAVR found that end-stage renal disease and coagulopathy were the most significant predictors of major bleeding.21 A large, German single-centre analysis of patients undergoing TAVR with third-generation devices, as well as the Perclose ProGlide® system (Abbott Vascular, Chicago, IL, USA), found that higher sheath-to-femoral-artery ratio and more than two vessel entries with large-bore sheaths were significant predictors of access-related vascular complications (p<0.001 and p=0.029, respectively).22
Recently, a TAVR-specific bleeding risk model was developed and validated with the patient populations of two large European registries; in this study, machine learning selected six variables to generate a bleeding risk score: blood haemoglobin, serum iron, creatinine clearance, common femoral artery (CFA) diameter, oral anticoagulation and dual antiplatelet therapy.23 External validation of this model for bleeding events through 30 days showed good performance, with the area under the receiver-operating characteristic curve of 0.78 (95% confidence interval 0.72–0.82; p<0.001). Though there are distinct differences between patients undergoing TAVR and those undergoing pLVAD-supported HRPCI, with TAVR patients generally being of older age and women in greater percentage, risk factors identified with TAVR should be noted by interventionalists performing HRPCI with large-bore access.
Access strategy
Ultrasound guidance for femoral puncture
Ultrasound guidance in addition to conventional fluoroscopic guidance in patients at high risk for vascular complications appears to reduce complications. While the FAUST randomized controlled trial, which assessed ultrasound versus fluoroscopic guidance for femoral access, failed to show an overall difference in CFA rates (the primary endpoint), ultrasound guidance resulted in significantly reduced rates of vascular complications (1.4% versus 3.4%; p=0.04), as well as fewer attempts, more successful first passes, lower risk of inadvertent venipuncture and shorter time to access.24
The reduced number of attempts observed with ultrasound guidance is likely directly linked to the reduced number of vascular injuries seen with this approach. Gabriel et al. found that pseudoaneurysm formation occurred in 2.6% of patients with access obtained under ultrasound guidance compared with 4.5% of patients undergoing palpation-guided access.25 The authors further note that pseudoaneurysm formation was dependent on the artery punctured: if the external iliac artery, superficial femoral artery or deep femoral arteries were inadvertently punctured, the risk for pseudoaneurysm formation increases dramatically. This risk can be limited with ultrasound mapping of the peripheral arterial tree. Particular care to avoid a low femoral puncture below the CFA bifurcation, particularly in patients who are underweight or obese, also reduces this risk. Low femoral puncture has been identified as the strongest risk factor for pseudoaneurysm development;26 the use of ultrasound to localize the CFA head limits this risk.27 In addition, routine use of ultrasound has also been associated with substantial reductions in access-related vascular and bleeding complications for TAVR.28–30
Micropuncture
In a large retrospective analysis of 17,844 patients undergoing PCI at a single institution over 8 years, Ben-Dor et al. found that patients undergoing PCI access with a Micropuncture® needle (Cook Medical, Bloomington, IN, USA) had significantly lower rates of access-site complications compared with those undergoing access with standard needles (2.5% versus 3.6%; p=0.005).31 This reduction in overall access complications (particularly haematoma) using Micropuncture needles occurred despite the higher-risk patient profile of this group, including a significantly higher percentage of anticoagulation (23.7% versus 10.2%; p<0.001).
Vessel preparation
In some patients with significant PAD, the transfemoral passage of a 12 to 14 French (Fr) sheath may not be feasible because of vessel rigidity and/or tortuosity. Intravascular lithotripsy (IVL) is a recently approved vessel preparation technique in which the Shockwave device (Shockwave Medical Inc., Santa Clara, CA, USA) delivers pressure waves that fracture calcified lesions, greatly enhancing vessel compliance and allowing femoral access in patients who otherwise would be anatomically ineligible.32 The risk of vessel injury with this technique is minimal, as the pressure waves are deployed on calcific lesions, sparing surrounding tissue; furthermore, the inflation pressures required are low.33 IVL, as vessel preparation, facilitates transfemoral delivery of large sheaths, including TAVR systems.34 Reported IVL experience with pLVAD insertion is still limited but growing.35–37 In a recent retrospective, multi-centre case series of 12 patients successfully treated with IVL to facilitate femoral access for pLVAD placement, the use of IVL avoided alternative (transcaval or transaxillary) access or unsupported HRPCI.37 In half of the cases considered, femoral delivery of the pLVAD had been attempted unsuccessfully prior to use of IVL; in the other half, IVL preceded balloon angioplasty.
The importance of learning/maintaining femoral skills
While femoral access has been the primary access site, radial access has been growing in use due to less frequent access complications, improved outcomes in acute coronary syndrome and ST-segment elevation infarction procedures, and shorter hospital stays.38,39 New operators are principally trained in radial access, an approach first reported by Lucien Campeau in 1989,40 with little experience in femoral access. As a result of this, the safety benefits observed with the radial approach are offset by a paradoxical increase in vascular access complications when femoral access is used; this is termed the Campeau Radial Paradox.41 Though radial access may be the first choice for routine PCI, femoral access is still needed in certain emergent or anatomically complex PCI cases and for large-bore pLVAD sheath procedures. Thus, operators with limited femoral skills or training in femoral access can reduce complications from pLVAD access with updated femoral training or retraining. Recent training recommendations for HRPCI highlight the importance of femoral access, closure and complication management training, with a suggested 100 ultrasound-guided femoral access procedures and a minimum of 15 procedures with MCS support during training.42 Thus, femoral access training and retraining are important for minimizing access-site complications and bleeding.
Vascular closure techniques and devices
Vascular closure devices (VCDs) can be a significant component of a successful bleeding avoidance strategy for large-bore access. A CathPCI Registry analysis of bleeding avoidance strategies in >1.5 million US patients found that patients treated with VCDs had lower rates of bleeding complications compared with those treated with manual compression (2.1% versus 2.8%; p<0.001), particularly those at a high risk of bleeding (4.6% versus 6.1%; p<0.001), and further improved when VCDs were combined with bivalirudin (0.9% in all patients, 2.3% in high-risk patients).43
VCDs allows for significantly earlier haemostasis and ambulation, as well as greater levels of patient satisfaction compared with manual compression.44 For patients with hypertension, in whom it may be more difficult to achieve haemostasis via manual compression, VCDs show a clear benefit and significantly reduced complications.45 A downside to VCDs is that their failure rate is highly variable and may depend on operator experience.46 Furthermore, when VCD failure does occur, it is associated with a significantly higher vascular complication rate.47 Evidence supporting the effectiveness of currently available VCDs for large-bore sheath procedures, such as TAVR and pLVAD placement, is less robust, with heterogenous outcomes.48–52
Some operators recommend large-bore sheath removal in the catheterization laboratory using a dry closure technique, in which a balloon is inflated proximal to the access site, at which point the sheath is removed and a Perclose ProGlide system deployed.53 Careful observation for bleeding post-sheath removal is important, as the balloon tamponade can be reinstituted while repairing the closure site.
The new collagen-based MANTA™ VCD (Teleflex, Morrisville, NC, USA) was approved by the US Food and Drug Administration in 201954 and is designed specifically for large-bore (10–20 Fr) femoral access site closure, available in 14 or 18 Fr versions. In the limited number of studies with this novel device, the MANTA VCD has shown improved outcomes compared with currently available suture-based VCDs.55–58 However, the recently published MASH randomized controlled trial, which compared MANTA to two ProGlides (Abbott Vascular, Abbott Park, IL, USA) in patients undergoing TAVR, reported a similar performance on the primary endpoint of access site-related vascular complications between the two devices, MANTA and the ProGlides.59 However, patients treated with MANTA had a significantly lower rate of modified VCD failure (defined as VCD failure to achieve haemostasis within 5 minutes or requirement for additional endovascular manoeuvres, such as stenting, surgery or additional VCDs), and a 20% modified VCD failure rate compared with 40% with the two ProGlide devices (p=0.002). Patients undergoing the MANTA VCD also had a significantly shorter time to haemostasis.
In a US pivotal study (16% of patients treated with the 14 Fr MANTA and 84% with the 18 Fr MANTA), technical success was achieved in 97.7% of patients, and Valve Academic Research Consortium 2 complications occurred in 4.2% of patients, with the authors speculating that more frequent use of the 14 Fr MANTA may be associated with lower vascular complication rates.60 A recent single-centre study assessed the MANTA VCD in 22 patients receiving MCS for HRPCI (68%) or cardiogenic shock (32%), with a majority receiving pLVADs.61 Technical success was reported in 96% of patients. These results were achieved despite constituting the first experience for most operators. Larger, randomized studies using the MANTA VCD specific to pLVADs will be important. The published evidence shows that the learning curve with this device may be shorter than has been observed with suture-based closure systems. Currently, reimbursement limitations may discourage widespread use.
Large-bore access learning curve
In the USpella registry, transfusion rates decreased sequentially from 12.2% in 2009 to 6.1% in 2011,62 which was attributed to a learning curve with large-bore closure techniques. In a subanalysis of the PROTECT II study, Henriques et al. found that the 30-day and 90-day major adverse events rates were substantially higher for the first patients treated at each site compared with the remaining patients.63 While no studies have quantified a pLVAD learning curve, given that TAVR studies have identified dramatic learning curves for <200 cases,64,65 it is likely that greater experience with the procedure will be associated with fewer complications.
Single access
Using a single-access technique for PCI access via the Impella sheath appears to reduce access site complications by reducing the number of access sites.66 The concept is to puncture the Impella sheath valve leaflets, inserting a 6 Fr up to a maximum of 7 Fr hydrophilic PCI sheath for the guide catheter.66 Wollmuth et al. reported no vascular complications for single access in 17 patients, except for one patient who developed iliac thrombosis when the Impella was not removed in the catheterization laboratory.66 Though evidence for the safety and efficacy of the single-access approach is still limited, this technique shows promise as a means to reduce access site complications. While still preliminary, the small case series that have been published are encouraging.66–70
Alternative access sites: axillary access
Recently, percutaneous axillary access has been introduced as an alternative access site in the setting of severe PAD limiting conventional femoral access. Although alternative access is often possible via surgical cutdown of the femoral, subclavian or axillary arteries, patients with substantial aortoiliofemoral disease, contraindicating the femoral approach, often have considerable comorbidities, potentially introducing the added risks associated with general anaesthesia.71 While the axillary artery is generally smaller in diameter than the CFA, calcification of the axillary artery was found to be much less frequent (1–2%) than the CFA (18–20%) based on Tayal et al.’s analysis of 110 computerized tomography scans.71 Axillary access may also be preferred in patients who are morbidly obese to decrease bleeding risk or in patients with highly tortuous or small iliofemoral arteries. In addition, axillary access may be preferred in patients who may require prolonged MCS, as this allows for improved patient comfort and mobility while on support.
The largest study to date assessing the feasibility of axillary access for pLVAD insertion included in 48 patients (60% presenting with cardiogenic shock) in whom femoral MCS placement was contraindicated.72 The technique requires an angiographic assessment of the axillary artery to accommodate a large Impella sheath, Micropuncture access and double-Perclose closure (off-label in axillary arteries), although balloon tamponade was used as per operator discretion in cases where the Perclose was deemed unlikely to provide a successful closure. With this approach, the authors reported a learning curve in terms of access time, but axillary access required less pLVAD repositioning. No vascular complications occurred with the percutaneous axillary technique, but one patient (2.1%) suffered a stroke. A recent systematic review of a percutaneous compared with surgical transaxillary approach for TAVR or pLVAD insertion in a predominantly cardiogenic shock population demonstrated similar rates of mortality, stroke and vascular complications; however, significantly less bleeding occurred with the percutaneous approach.73
Several studies have demonstrated a higher incidence of stroke in large-bore subclavian/axillary access for TAVR compared with traditional femoral access, which may be related to the access itself or to the inherent stroke risk in patients with a degree of vascular disease that ultimately precluded them from femoral access.74–77 Given this concern for neurologic sequelae, axillary access should only be considered in patients lacking traditional femoral access in whom revascularization with MCS is viewed as the best treatment option.
Post-procedural care
Most patients undergoing pLVAD-supported HRPCI do not require extended support or anticoagulation. Early pLVAD removal following PCI is optimal for reducing the risk of bleeding complications. A cVAD registry analysis of patients with immediate pLVAD removal post-PCI compared with extended use of acute MCS found that the group who received extended Impella support after PCI had a significantly higher incidence of vascular complications and death.78
The non-physician healthcare professionals who provide periprocedural care play an important role in both preventing and managing post-PCI access-related complications. At some centres, the non-physician staff may be responsible for sheath removal and manual compression to achieve haemostasis. While a Dutch study found that registered nurses achieved good performances for sheath removal post-PCI, the authors recommended yearly repetition of training and observation to enhance and maintain skills, emphasizing the adherence to coagulation parameters regarding the timing of sheath removal.79 However, the management of sheath removal and manual compression for haemostasis by non-physician staff may not be applicable to large-bore access.
A recent Chinese study randomly assigned patients with acute myocardial infarction undergoing emergent PCI to receive either conventional nursing care or nursing care according to a clinical nursing pathway (CNP) protocol using the model from the Clinical Pathways for Acute Coronary Syndrome II study.80 The authors found that there was a significant reduction in postoperative complications, length of stay and hospitalization costs with the CNP approach. Subcutaneous haemorrhage occurred in 4.8% of patients undergoing CNP compared with 16.1% of those undergoing conventional care, while abdominal pain and bleeding occurred in 4.8% versus 19.6% of patients. Patient satisfaction was also significantly higher with the CNP approach. Thus, institutions need a standard protocol with regular training/retraining for out-of-catheterization laboratory sheath removal to achieve optimal outcomes.
Anticoagulation strategy
The anticoagulation strategy for pLVAD-supported HRPCI has some unique challenges. The pressure and flow rate of the unfractionated heparin (UFH) purge solution for Impella pumps varies depending on flow resistance. Consequently, the amount of UFH added to the systemic circulation fluctuates over time but must be accounted for in calculating total systemic intravenous heparin administration. Reed et al. conducted a survey-based study assessing anticoagulation practices with Impella for cardiogenic shock or HRPCI at centres performing more than one Impella case per month.81 Across 65 responding centres, 83.1% were using the recommended purge concentration with 5% dextrose in water, but only 52.4% of centres used the recommended UFH concentration for the purge solution (50 units/mL). For patients with heparin-induced thrombocytopenia, only 25.0% of centres had a dextrose-only strategy, as recommended by the manufacturer, while 16.7% of centres had no protocol. Centres also varied widely on other considerations, such as intravenous heparin adjustment for initial purge flow (performed by only 59.0% of centres), and timing of intravenous heparin initiation (37.7% of centres at the time of device insertion and 49.1% when patients were subtherapeutic on the purge solution alone). Importantly, 56.7% of centres were using activated partial thromboplastin time as opposed to the activated clotting time recommended by the manufacturer (21.7% of centres).
A recent study comparing the outcomes of patients undergoing HRPCI with Impella support in the PROTECT III trial (2017–2020) versus the historic PROTECT II trial (2007–2010) found a significant reduction in bleeding complications requiring transfusion in the PROTECT III patients (1.8% versus 9.3%; p<0.001).82 The authors speculated that this reduction may have been due, in part, to the shift away from glycoprotein IIb/IIIa inhibitors, due to their association with elevated bleeding rates, resulting in their significantly lower use in PROTECT III.83 The more prevalent use of VCDs in recent years was also posited as a potential explanatory factor, in addition to the impact of best practices learned and implemented from a decade of use with these devices for supported HRPCI.
Multiple factors affect bleeding risk. Some patients may have already been on prolonged anticoagulants, further elevating the risk of bleeding during PCI. In addition to the risk of UFH, concomitant use of dual antiplatelet therapy adds to bleeding risk. With elective MCS HRPCI, every effort should be made to minimize baseline anticoagulation before starting a procedure. In a nurse-led study of vascular access complications in patients undergoing cardiac catheterization procedures, aggressive anticoagulation was identified as a significant factor for vascular access complications.84 The optimal anticoagulation protocol is beyond the scope of this report and must, by nature, vary across institutions. However, the improvement of patient outcomes relies on the development of consistent, centre-specific anticoagulation protocols focused on effective but safe levels of anticoagulation based on manufacturer recommendations but modified as needed for local circumstances.
Conclusions
Standardized protocols and continued training and experience to optimize access, closure, anticoagulation management and post-procedural care should continue to reduce the risk of access-related bleeding and vascular complications for pLVAD-supported procedures. Coupled with evolving technological advances, improving outcomes for large-bore access markedly limits vascular access risk as a barrier to appropriate pLVAD use in HRPCI.