The current outbreak of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has developed into a worldwide pandemic affecting millions of lives in one way or another.1 Over the period of 1 year since the disease was first reported from Wuhan, China,2 COVID-19 has infected over 50 million patients and resulted in nearly 1 million deaths at time of writing.3 COVID-19 predominantly affects the respiratory system, causing acute respiratory distress syndrome in severely affected patients, which can result in death from respiratory failure. Cardiovascular complications, such as myocarditis, heart failure, acute coronary syndromes (ACS), pulmonary embolism, stress cardiomyopathy and arrhythmias, have been described in these patients.1 One of the major complications of COVID-19 is cardiac arrhythmias, which have often been considered a marker of poor prognosis. Both brady- and tachyarrhythmias have been described in such patients. In a report from China concerning patients with COVID-19, around 17% of admitted and 44% of patients in the intensive care unit (ICU) were found to have cardiac arrhythmias.4 To date, our understanding regarding the arrhythmogenicity in COVID-19 is still evolving. In this article, we discuss the incidence, pathophysiology, various types and management of cardiac arrhythmias in patients with COVID-19.
Pathophysiology of arrhythmias in COVID-19
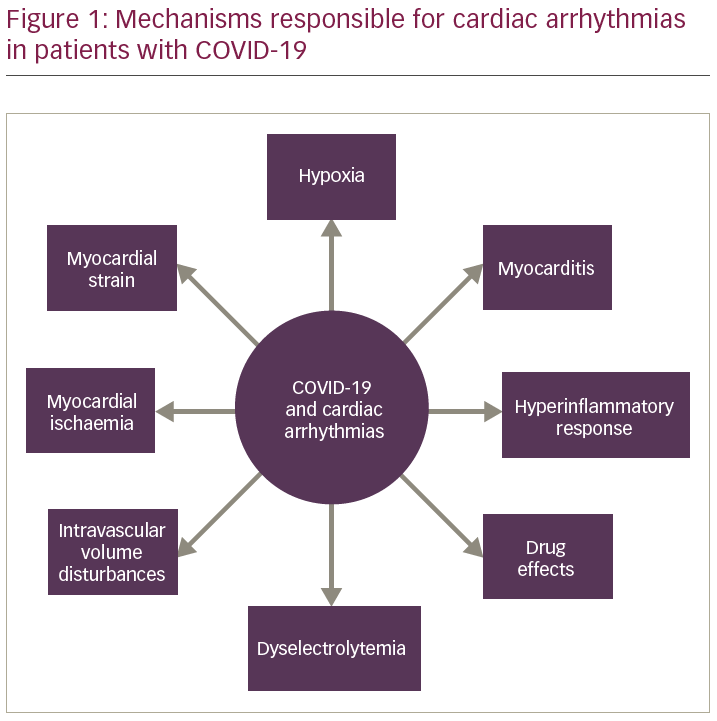
The aetiology of arrhythmias in COVID-19 is multifactorial. Arrhythmias may occur due to myocardial damage associated with COVID-19 (myocarditis, ACS, Takotsubo cardiomyopathy), or as an indirect manifestation of hypoxic injury, metabolic disturbances, myocardial strain and neurohormonal imbalance (Figure 1).5 Cardiac injury is common in patients with COVID-19, which has attracted significant attention in the medical community.1 Huang et al. reported myocardial injury (defined by a rise of cardiac biomarkers above the 99th percentile upper reference limit) in 12% of patients with COVID-19.6
SARS-CoV-2 is internalized into the cardiomyocyte after binding to angiotensin-converting enzyme-2 (ACE-2) surface receptors on cell membranes, following which, the virus starts replicating using the host cell’s molecular machinery, such as the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) pathway.1 The activated NF-kB, in turn, affects the messenger RNA (mRNA) expression of various pore-forming subunits for transient outward potassium current, potentially inducing arrhythmia by affecting the action potential of the cell. SARS-CoV-2 can also induce cellular death by upregulating the expression of genes related to cluster of differentiation 40 (CD40), caspase recruitment domain family member 8 (CARD8), and serine/threonine kinase 4 (STK4). This myocardial damage can cause electrical abnormalities and induce arrhythmias. Besides, myocarditis can also be postulated as one of the potential causes for cardiac arrhythmias. The direct pathologic effect of SARS-CoV-2 on cardiac myocytes or cell-mediated cytotoxicity by CD8+ lymphocytes can be proposed as the probable mechanisms of myocarditis in COVID-19.5,7 Arrhythmias in myocarditis can occur either due to a direct cytotoxic effect, or following ischaemia from microvascular damage or gap junction dysfunction due to impaired connexin expression.5,8 There have been reports of fulminant COVID-19 myocarditis being associated with cardiac arrhythmias, with diagnosis established on cardiac biopsy/cardiac magnetic resonance imaging.9–11 Hypoxia, caused by respiratory failure in COVID-19, can promote cardiomyocyte cell death and affect the functioning of ion channels.1,5 This leads to alterations in cardiomyocyte action potential, thereby enhancing arrhythmogenesis. Prolongation in ventricular repolarization may lead to re-entrant arrhythmias; shortening of the repolarization time also may be pro-arrhythmic.7
COVID-19 infection is often characterized by a cytokine storm, with an elevation of various cytokines, such as interleukin-6 (IL-6) and interleukin-1β (IL-1β), especially in critically ill patients.1 IL-6 has been found to cause atrial electrical remodelling by downregulating atrial myocyte junction proteins, which affects the cardiac action potential. Other cytokines, such as IL-1β, reduce Ito current, IL-2 increases peak INa density, tumour necrosis factor-alpha decreases IKr density in ventricular myocytes; all of which can induce arrhythmia.5
Fever is a common symptom among patients with COVID-19. In some cardiac diseases affecting ion channels, such as Brugada syndrome, fever can trigger malignant ventricular arrhythmias.12 SARS-CoV-2 inhibits the activity of ACE-2 after binding to ACE-2 surface receptors by shedding or internalization of ACE-2 from cell membranes, which in turn decreases the conversion of angiotensin II to angiotensin-(1–7).13 The protective effect of angiotensin-(1–7) through the Mas receptors is thus reduced. Also, the increased activity of angiotensin II results in the induction of nicotinamide adenine dinucleotide phosphate oxidase 2 leading to oxidative activation of Calmodulin kinase II, further promoting sarcoplasmic reticulum-Ca2+ leakage, thereby increasing the possibility of delayed afterdepolarisation.14
In the initial days of the pandemic, a great deal of controversy was generated on the putative role of ACE inhibitors/angiotensin-receptor blockers (ARBs) in increasing the risk for COVID-19 infection. This stemmed from the fact that SARS-CoV-2 binds to the target cells through ACE2 receptors. The proponents of this theory postulated that there was an upregulation of ACE2 receptors by ACE inhibitors and, therefore, a greater number of receptor sites available for SARS-CoV-2 to bind to.15 However, further studies showed that this theory did not hold up to scrutiny and the binding of SARS-CoV-2 to ACE2 leads to downregulation of the ACE2 receptors, thus decreasing the available binding sites.13 Also, the other deleterious aspects of the use of ACE inhibitors was inhibition of ACE2 by ACE inhibitors. ACE2 is responsible for the breakdown of angiotensin II to angiotensin-(1–7), thus decreasing the effects of angiotensin II such as vasoconstriction, sodium retention and fibrosis.13 However, it was seen that ACE and ACE2, despite having a structural similarity, have varying enzymatic sites; hence, there is no role of ACE inhibitors on ACE2 activity.13 As a result, it was proved beyond doubt that ACE inhibitors/ARBs are safe and may have a beneficial effect.1,13
The other potential mechanisms responsible for cardiac arrhythmias in these patients with COVID-19 include myocardial strain and intravascular volume disturbances.5 Right myocardial strain in patients with COVID-19 occurs either due to (1) acute pulmonary embolism, often as a result of a thrombotic complication, or (2) due to pulmonary hypertension following sepsis, acute respiratory distress syndrome, or left-sided heart failure. This leads to higher right atrial pressures, which, in a background of increased sympathetic tone as seen in critically ill patients, often predisposes to atrial tachyarrhythmias.5 Intravascular volume disturbances are often seen in critically ill patients with COVID-19 as a result of sepsis or heart failure. In such clinical scenarios, atrial tachyarrhythmias – especially atrial fibrillation (AF) – is often encountered.16
Arrhythmias encountered in COVID-19
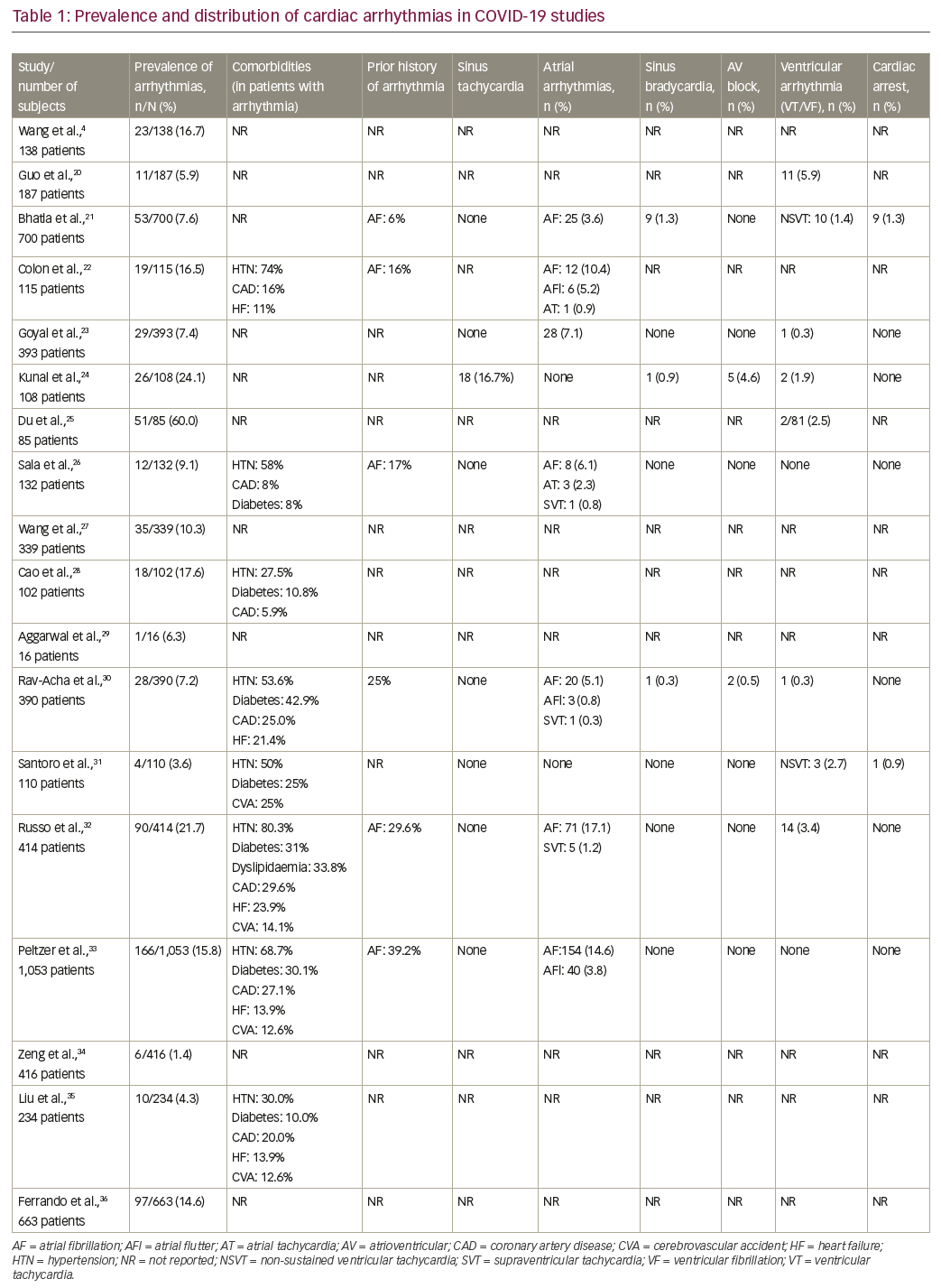
Data from the previous SARS and the Middle East respiratory syndrome (MERS) epidemics show that arrhythmias were one of the most important cardiovascular complications.17,18 In a series of 121 patients with SARS, 71.9% had tachycardia, while 14.9% had bradycardia.17 Similarly, in a series of 70 patients with MERS, cardiac arrhythmias occurred in 15.7%.18 Other viral infections, such as influenza, have also been noteworthy in causing tachyarrhythmias, heart block and ventricular fibrillation (VF), often secondary to myocarditis.19 Currently, limited data exist regarding arrhythmic complications in COVID-19 (Table 1).4,20–36 Arrhythmias frequently encountered in patients with COVID-19 include supraventricular tachycardia (SVT), atrial flutter, AF, complete heart block, monomorphic/polymorphic ventricular tachycardia (VT) and multifocal VT.5 Wang et al. highlighted that arrhythmias were reported in 17% of hospitalized patients.4 In one of the larger studies involving 700 patients with COVID-19, there were 25 new-onset AF events, 9 clinically significant bradyarrhythmias, and 10 non-sustained VTs (NSVTs).21 The authors concluded that cardiac arrhythmias and cardiac arrests are mostly the consequence of systemic illness and not solely direct manifestations of SARS-CoV-2 infection.21
Tachyarrhythmias
Sinus tachycardia is the most common arrhythmia among patients with COVID-19 and reflects the heightened sympathetic tone in acute stages of infection.37 Pathologic arrhythmias in patients with COVID-19 include AF, atrial tachycardias and NSVTs.1,24 Colon et al. reported new-onset atrial tachyarrhythmias including atrial tachycardia, atrial flutter and AF in 16.5% of hospitalized patients.22 Goyal et al. also reported that atrial arrhythmias were more common among patients with COVID-19 requiring mechanical ventilation (17.7% versus 1.9%).23 In a worldwide cross-sectional survey by the Heart Rhythm Society (HRS), the most commonly reported tachyarrhythmia was AF.30 AF can be secondary to sepsis, hypoxemia, virus-mediated cardiomyocyte injury or an increased susceptibility due to advanced age and comorbidities. In a Chinese cohort, Guo et al. reported that 5.9% of patients with COVID-19 had ventricular tachyarrhythmias.20 In a survey among 1,197 electrophysiology professionals by HRS, ventricular premature complexes (VPCs) were most commonly reported ventricular arrhythmias (monomorphic VPCs: 5.3%; polymorphic VPCs: 3.5%), followed by NSVT (6.3%). Monomorphic VT was reported by 3.8% of respondents, polymorphic VT/torsades de pointes (TdP) by 3.5%, VT/VF by 4.8% and pulseless electrical activity by 5.6%.38 Ventricular arrhythmias in COVID-19 are usually encountered in the setting of acute myocardial injury and myocarditis. Studies have reported acute myocardial injury in 5–38% of patients with COVID-19, which can be one of the inciting factors for the occurrence of ventricular arrhythmias.39 In a retrospective study of 1,284 patients, among the 170 subjects with cardiac injury, arrhythmias were reported in 25.9% patients, with six patients developing VT/VF – all of which were fatal episodes.40
Bradyarrhythmias
Bradyarrhythmias, such as sinus bradycardia, sinus node dysfunction and complete heart block are all commonly encountered among patients with COVID-19.7 Data from the previous SARS pandemic reported sinus bradycardia in up to 15% of patients.17 A host of aetiologies, including myocarditis, sepsis and the adverse effects of drugs, are responsible for the occurrence of bradyarrhythmias.5 Drugs such as chloroquine and hydroxychloroquine affect the conduction system.41 Studies involving long-term chloroquine use suggest an increase in the refractory period of Purkinje fibres and action potential duration, culminating in atrioventricular nodal and infra-Hisian conduction block.41 In a series of seven patients with COVID-19 and bradyarrhythmias, five had complete heart block, while two had sick sinus syndrome.42 In another series of seven patients with COVID-19, bradyarrhythmias served as a poor prognostic marker with higher short-term morbidity and mortality, despite prompt treatment with cardiac pacing.43
Drug-induced arrhythmias
Several COVID-19 therapies, including repurposed older drugs such as hydroxychloroquine, chloroquine, lopinavir/ritonavir and azithromycin, are known to increase the risk of TdP due to QT prolongation, especially in critically ill patients with COVID-19.1 The baseline QTc interval needs to be determined before administration of these drugs.1 In a multicentre study from New York, 6.1% of patients with confirmed COVID-19 were detected to have a baseline QTc interval >500 milliseconds (ms) at the time of admission.44 These patients should be identified with dose modification of the drugs, along with close monitoring of the QTc interval as they have a higher risk of TdP. Many drugs in isolation, as well as in combination, pose a significant risk of QTc prolongation and TdP. Both chloroquine and hydroxychloroquine, being structurally similar to quinidine, show QT-prolonging effects by blocking the activation of potassium channel IKr (hERG/Kv11.1).45,46 Also, these drugs are metabolized by cytochrome P450 3A4 (CYP3A4), hence co-administration of other medications that inhibit CYP3A4 enzyme, such as statins, may result in elevated plasma levels of chloroquine and hydroxychloroquine.45
Borba et al. compared the effect of high versus low doses of chloroquine in patients hospitalized with COVID-19.47 They reported enhanced toxic effects and lethality, particularly affecting QT prolongation among patients treated with a higher dosage of chloroquine.47 This study suggests that higher doses of chloroquine increase the propensity for QTc prolongation, arrhythmias and mortality. In another study involving 84 patients with COVID-19 being treated with hydroxychloroquine and azithromycin, a severe QTc interval prolongation (>500 ms) was detected in 11% of patients. However, none of them were detected to have TdP events.48 Similarly in a retrospective cohort of 90 patients with COVID-19, administration of hydroxychloroquine with or without azithromycin had an increased the risk of QTc prolongation and further heightened the risk of TdP.49 In a series of 108 patients, among the patients who had received both hydroxychloroquine and azithromycin therapy, a significant prolongation of QTc was observed (452.5 ± 41.7 ms versus 427.2 ± 22.6 ms; p=0.003), compared with those who received hydroxychloroquine alone. Prolongation of QTc was reported in 23.9% of patients who had received both hydroxychloroquine and azithromycin, compared with a single patient (3.5%) with hydroxychloroquine alone (p=0.009).24
Clinical presentation
A greater proportion of patients with COVID-19 do not have symptoms pertaining to arrhythmias or conduction system disease.50 This makes it imperative to follow sick patients closely, with cardiac rhythm monitoring for early recognition of arrhythmic changes. Tachycardia in these patients may be secondary to systemic causes, such as fever, pain, respiratory distress and underlying anaemia.37 The manifestations of arrhythmia in COVID-19 may vary from an asymptomatic presentation to sudden cardiac death. Palpitation as a presenting symptom has been reported in 7.3% of patients.51 Also, these patients may present with unexplained syncope or as survivors of sudden cardiac arrest.
Increasing age is, in itself, a major risk factor for arrhythmias such as AF in these patients.52 The frequency of comorbidities in patients with arrhythmias in various studies among patients with COVID-19 has been highlighted in Table 1.21,22,26,30,32,33 In a study comprising 1,053 patients, those with arrhythmias were older, with a significantly higher frequency of comorbidities.53 An important challenge faced by clinicians is to determine whether the patient has a new onset arrhythmia or a pre-existing one. Around 6–39% of patients with COVID-19 have a previous history of cardiac arrhythmia, which are mostly episodes of AF (Table 1).21,22,26,30,32,33 These patients are often at increased risk for cardiac arrhythmias and hence require a greater degree of vigilance.
Diagnostic strategies
Electrocardiogram
On hospital admission, a baseline electrocardiogram (ECG) should be performed, especially in patients with a moderate to severe disease or in whom QT-prolonging medicines are being considered.54 This allows for detection of myocardial injury and ACS, and documentation of baseline QTc interval. Ideally, this should be a 12-lead ECG strip; however, a single- or three-lead ECG strip from telemetry monitoring or a hand-held ECG device may be adequate to minimize the post-exposure hazard to healthcare personnel. The differentiation between various tachycardias can be made with a 12-lead ECG based on regularity, QRS width and atrioventricular dissociation, among other characteristic features. A single ECG tracing might not be comprehensive and often dynamic ECG monitoring is required to identify various arrhythmias. Acquired QT prolongation can be detected by measuring QTc on a 12-lead ECG, in association with QT-prolonging drug therapy or a clinical scenario known to cause QTc prolongation.55,56 A QTc of >500 ms is considered highly abnormal for both males and females, which also holds to be true among patients with COVID-19.56 Importantly, many hospitalized patients would need QTc monitoring to reduce the risk of QTc prolongation and TdP if QT-prolonging medications are initiated.1
Continuous electrocardiographic monitoring
In stable patients with minimal symptoms, continuous ECG monitoring is not required in the absence of a documented arrhythmia or suspected myocardial ischaemia.5 However, in patients admitted to ICU settings, continuous ECG monitoring in conjunction with automated blood pressure readings and oxygen saturation monitors (instead of periodic vital signs monitoring by nursing staff) is essential to detect arrhythmic and other systemic complications at the earliest.5 This practice also reduces the number of healthcare staff interacting with the patient, thereby reducing the risk of exposure hazard and preserving personal protective equipment.5
Echocardiogram
Cardiac evaluation with an echocardiogram is not necessary for all patients with COVID-19. However, patients with suspected myocardial injury or ACS should undergo echocardiographic examination, taking all the necessary protective measures against cross-infection.1 An echocardiogram can identify features suggestive of myopericarditis, ACS and Takotsubo cardiomyopathy (e.g. global or regional ventricular wall motion abnormalities, lower cardiac output, pericardial effusion and apical ballooning); all of which can aggravate the risk of cardiac arrhythmias, mandating intensive cardiac monitoring.1
Management strategies
Multiple cardiological societies, including the European Society of Cardiology, Latin American Heart Rhythm Society and the Mexican Societies of Cardiac Electrophysiology, have published a consensus document for the management of arrhythmias in patients with COVID-19.57,58 Since these arrhythmias are transient and associated with drug–drug interactions, aggressive management is often not warranted. It must be highlighted that the focus must be on the prevention of cardiac arrhythmias, especially in critically ill patients. Currently, the guidelines do not advocate prophylactic antiarrhythmic agents in patients with COVID-19.57,58
Management of polymorphic ventricular tachycardia and torsades de pointes
All patients presenting with TdP should be immediately assessed for symptoms, vital parameters and consciousness level to determine haemodynamic stability.38 Patients with sustained TdP usually become unstable and may collapse rapidly; they should be resuscitated as per the standard protocols and algorithms of advanced cardiac life support, including cardiac defibrillation.59 Intravenous magnesium sulphate, anti-tachycardia pacing and intravenous infusion of isoproterenol (rate: 1–4 μg/min, target heart rate: 100/min) are useful for managing TdP. Intravenous magnesium is often administered at a dose of 2 g, followed by a repeat dose 5–15 minutes later. Alternatively, an infusion of magnesium sulphate can be started at a dose of 1–4 g/h to keep serum magnesium levels >2 mmol/L.59 Patients may remain haemodynamically stable with repetitive outbursts of TdP intermittently. In patients with a single episode, correction of metabolic/electrolyte derangements and removal of inciting medications should be done along with intravenous magnesium therapy.5,59 These patients should have continuous cardiac monitoring until electrolytes and QTc are normalized.5,59 For patients having multiple self-terminating episodes of TdP, the same therapies must be instituted along with other interventions to increase heart rate, such as overdrive pacing and/or intravenous isoproterenol infusion (contraindicated in patients with congenital long QT syndrome).59
Monitoring for QT prolongation
Before administration of any drug with the potential to prolong QTc, a patient’s baseline QTc should be obtained. Data from recent studies suggest a modest increase in QTc (20–30 ms) when treated with one or more QT-prolonging drugs, although the individual response may vary.60,61 Patients with baseline QTc >500 ms are at heightened risk of polymorphic VT, hence caution is needed in such patients.62 The presumed benefits and risks of such drug therapy should be weighed against each other before the initiation or continuation of these drugs to treat COVID-19. Also, an effort should be made to correct any electrolyte disturbances that could potentially increase the risk of arrhythmias, such as hypokalaemia, hypomagnesemia and hypocalcaemia.1 Patients with COVID-19 who are hospitalized can be monitored with a 12-lead ECG (or single-/three-lead ECG to limit resource consumption and healthcare worker exposure); however, there is no clear guidance regarding monitoring of outpatients with COVID-19 who are receiving QT-prolonging therapies. Strik et al. attempted to validate QTc measurements in 100 patients with COVID-19 using the Apple smart watch (Apple Watch series 4; Apple Inc., Cupertino, CA, USA) single-lead ECG to enable remote monitoring during the COVID-19 pandemic.63 A single-lead ECG was recorded in three different locations (left wrist, left ankle and left lateral chest wall) using the Apple smart watch, and around 94% of patients were able to obtain an accurate QTc interval, which also correlated to 12-lead ECG. Therefore, novel rhythm monitoring techniques, such as the Apple smart watch, may have an incremental value in monitoring patients remotely, compared with traditional 12-lead ECG machines.
Protocols for monitoring QT interval
In accordance with a recent statement for monitoring of the QT interval in COVID-19, therapy with hydroxychloroquine or azithromycin/remdesivir/lopinavir/ritonavir can be initiated in a patient not on any QT-prolonging therapy, or with structural heart disease, bradycardia or long QT syndrome.45 A baseline ECG should be obtained in all patients with COVID-19. If QTc remains <500 ms without significant ventricular ectopics, therapy may be continued. If QTc is >500 ms or an increase in QTc is >60 ms on serial ECGs, a cardiology consultation should always be sought, with the decision to continue drug therapy considered on a case-by-case basis. There is an increased risk of TdP if ECG shows QTc >500 ms or a change in QT interval is >60 ms. All other QTc-prolonging medications should be discontinued along with prompt correction of electrolyte imbalances and the patient placed on a continuous cardiac monitor; external defibrillator patches or a wearable defibrillator should be considered if these are available.
The Mayo Clinic protocol also advocates for a baseline ECG in all patients planned for therapy with QT-prolonging drugs, such as hydroxychloroquine.56 In patients with baseline QTc >500 ms, it is imperative to avoid QT-prolonging drugs. However, at times, it may be reasonable to start therapy with these drugs when the benefits of hydroxychloroquine are greater than the risk of drug-induced sudden cardiac death. In these patients, QTc should be obtained after 2–4 hours following the first dose and then repeated at 48 hours and 96 hours. If there is further QT prolongation, therapy must be stopped, followed by serial ECGs and the monitoring of serum electrolytes. In patients with baseline QTc <500 ms, repeat ECG is advocated at 48 or 96 hours following drug initiation. If on therapy QTc >500 ms or delta QTc >60 ms, medications should be stopped along with the measurement of serum electrolytes.
In hospitalized patients, the risk for QTc prolongation can be determined using the Tisdale score, which is a composite of eight parameters, namely age >68 years, female sex, use of loop diuretics, potassium level <3.5 mEq/L, baseline QTc interval >450 ms, acute myocardial infarction, number of QT-prolonging medications, sepsis and heart failure.64 A Tisdale score <6 signifies a low risk for QT prolongation, 7–10 suggests medium risk, and a score >11 points towards a high risk of drug-associated QT prolongation.64,65 This score can also be used in patients with COVID-19 to risk-stratify them and identify those at higher risk for QT prolongation and intense monitoring.66 In a recently published series of 108 patients with COVID-19, a significant correlation was reported between Tisdale score and maximum QT interval (R=0.56; p<0.0001).24 This study also reported the optimal cut-off (>6) of Tisdale score in identifying patients with COVID-19 at risk of QT prolongation with a sensitivity of 85% and a specificity: 57% (area under the curve: 0.83; 95% confidence interval 0.74–0.93; p<0.0001).24
Prognostic significance of arrhythmias
Studies have shown that patients with COVID-19 and arrhythmias often have a poor prognosis.43,44,52,53 In a meta-analysis comprising four studies, arrhythmias were reported in 48% of patients who had poor outcomes.67 Similarly, a recent study highlighted that hospitalized patients who succumbed to their illness often experienced acute malignant cardiac arrhythmias (VT/VF or atrioventricular block) far more frequently than those who survived.68 Shao et al. studied the outcomes of in-hospital cardiac arrest retrospectively in a cohort of 136 Chinese patients.69 They reported that a vast majority of patients (87.5%) had a respiratory cause for their cardiac arrest. Of the resuscitated patients, the initial rhythm was non-shockable in the majority (89.7% asystole and 4.4% pulseless electrical activity), while shockable rhythm was present only in 5.9% of patients and a return of spontaneous circulation achieved in only 13.2% of patients. The 30-day survival was just 2.9%, and survival with intact neurologic function was 1%, showing poor survival among hospitalized patients who sustained in-hospital cardiac arrest.69
Conclusion
Cardiac arrhythmias are an important cardiovascular complication, especially among hospitalized critically ill patients with COVID-19 or those with multiple comorbidities. These arrhythmias are increasingly being encountered in patients requiring admission to a critical care unit and are often accompanied by haemodynamic instability. These often complicate the course of COVID-19 infection and are associated with a dismal prognosis. Therefore, early and prompt identification and management of these arrhythmias is required. Data regarding the occurrence of arrhythmias in patients with COVID-19 are sparse, with studies mostly involving sicker hospitalized patients during the acute phase of severe illness. As a result, the exact arrhythmic burden among patients with a milder form of disease or during recovery from COVID-19 infection is largely unknown. This calls for further large-scale studies to better understand the mechanisms underlying the cardiovascular system involvement and the arrhythmic burden in COVID-19, as well as to devise strategies for the management of arrhythmias associated with viral infections.