Introduction: The life expectancy of the general population has increased over the past few decades. This has led to a more elderly population and more frail patients requiring pacemaker implantation. It may be perceived by physicians, relatives and patients that pacemaker implantation in this cohort may be too invasive or have a higher complication rate. Data and outcomes of pacemaker implantation in nonagenarians are scarce. Herein, we report the outcomes of pacemaker implantation in a large number of nonagenarians at our institution.
Methods: We identified patients over 90 years of age who underwent initial pacemaker implantation between September 2017 and December 2021 at East Sussex Healthcare NHS Trust. All notes were retrospectively reviewed. The primary clinical endpoint was total mortality. The secondary endpoints included procedural characteristics and safety.
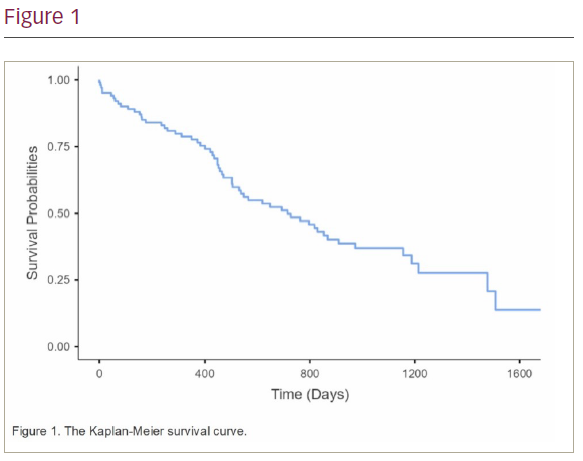
Results: A total of 100 patients were included. The average age was 93.50 ± 2.43. 57% of patients were female. Patients were followed up for an average of 611.58 ± 413.60 days. Overall, 82% of the implants were performed as an inpatient emergency; 69% of implants were due to AV nodal block, 19% due to sinus node dysfunction and 12% of implants were for rate control of atrial fibrillation. Axillary vein access was used in 62% of implants while cephalic vein and subclavian vein access were used in 35% and 3% of implants, respectively. The mortality rate during the follow-up period was 59%; 5% of patients died within 1 month of implant and 22% died within 1 year of pacemaker implantation (Figure 1). The complication rate was 4%. These included two pneumothoraces, one lead displacement requiring repositioning and one infected pacemaker requiring extraction.
Conclusion: The major complication rate in our cohort of patients was 4%. Although not insignificant, the complication rate is comparable to previous studies and registry data with younger cohorts. However, the majority of patients died within 2 years of pacemaker implantation. This is an important consideration when discussing the merits of pacemaker implantation in this cohort of patients. ❑