Tricuspid regurgitation (TR) is a complex disease with several independent causes. Consequently, outcomes and response to therapy vary widely. Severe or progressive TR often results in gradual right ventricular (RV) failure and is associated with poor long-term outcomes.1 Most cases of severe TR are secondary to left-sided heart disease, pulmonary hypertension or dilated cardiomyopathy, causing tricuspid valve (TV) annular dilation and leaflet tethering. However, study of distinct subtypes of TR, such as primary TR or that caused by atrial fibrillation, has highlighted that TR anatomy varies by aetiology. For example, chronic atrial fibrillation is associated with a greater degree of RV basal dilation and annular dilation compared with the typical pattern of RV elongation and leaflet tethering seen in other forms of secondary TR.2
Classically, patients with significant secondary TR have been managed with medical therapies directed at their underlying disorder and volume management with diuresis.3,4 Unfortunately, outcomes for patients with severe TR who are managed medically remain poor, with 5-year mortality approaching 50%.5 Additionally, the ideal timing of surgical repair or replacement of the TV remains unclear. The current American College of Cardiology/American Heart Association guidelines give a class Ia recommendation for repair of severe TV disease in patients already planned for left-sided surgery.1 However, recommendations for those with primary TR not planned for other cardiac intervention, prior left-sided surgery, asymptomatic TR or progressive but not severe TR remain less well defined.1 Many such patients are turned down for surgical repair or replacement given the high perioperative mortality, which can approach 25% in patients with prior left-sided surgery, at least in part due to RV failure at the time of operation.6
As such, investigations into less-invasive means of tricuspid repair have gained significant momentum, resulting in several innovative percutaneous, transcatheter approaches for repair and replacement of the TV. In this review we will focus on the current state of transcatheter TV therapies, and their outcomes, ongoing challenges and future directions.
Tricuspid anatomy
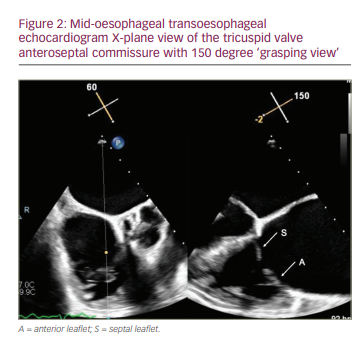
Understanding the anatomy of the TV is key to planning successful intervention. Both the tricuspid annulus and the TV apparatus itself have variable and complex anatomies. Historically, the TV was thought to comprise three leaflets – anterior, posterior and septal – however, up to 41% of patients studied with modern echocardiography have four or more functional leaflets, most commonly with two independent posterior leaflets.7 The anterior leaflet is typically the largest and most mobile, and in approximately 10% of cases, it is not fully separated from the posterior leaflet.8 The posterior leaflet is variable in size and in number of leaflet scallops. Comparatively, the septal leaflet is smaller and less mobile, with multiple chordae anchoring it to the right ventricle and RV septum.8
The anteroseptal commissure is highly conserved and is therefore an attractive anchoring target for percutaneous repair devices (Figure 1). This commissure typically lies adjacent to the non-coronary sinus of Valsalva and, in rare cases, can be a source of morbidity due to unintentional perforation of the aorta.8 Similarly, the atrioventricular (AV) node and His bundle pass within 5 mm posteriorly of the anteroseptal commissure and septal leaflet insertion, which can be damaged during TV intervention.8
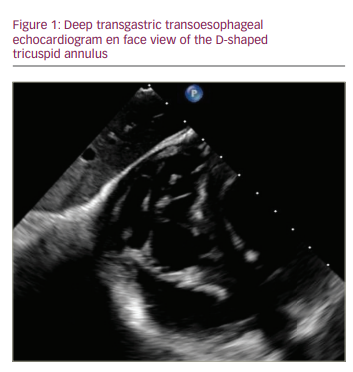
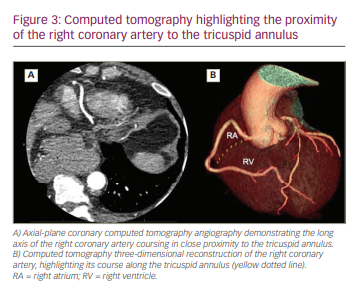
The tricuspid annulus differs significantly from the mitral annulus in that it is not a true, distinct fibrous annulus. It is largely devoid of fibrous tissue and is primarily composed of bands of endocardium and epicardium in a D-shape, with the curve of the “D” riding the RV free wall and the straight portion along the septum (Figure 2).9 The annulus itself is dynamic and can change in size up to 30% during the cardiac cycle, highlighting the challenge of performing percutaneous annuloplasty.10 In patients with severe TR, up to 40% have a right coronary artery (RCA) that courses within 2 mm of the tricuspid annulus, either anteriorly or posteriorly, increasing risk of perforation and making transcatheter annuloplasty in particular less favourable (Figure 3).11
Tricuspid valve assessment
Echocardiography is the primary modality for assessing TV anatomy and function, and RV characteristics. Although TR has historically been divided into three grades of severity, Hahn and colleagues have proposed an updated and detailed set of criteria that classifies TR into five grades based on transthoracic echocardiography (TTE).12,13 TTE not only indicates the severity of TR, but also reveals potential causes of TV disease, including anatomic variations, which cause primary TR, or left ventricular dysfunction, chamber dilation, upstream valvular disease or elevated estimated pulmonary pressures, which suggest secondary TR.
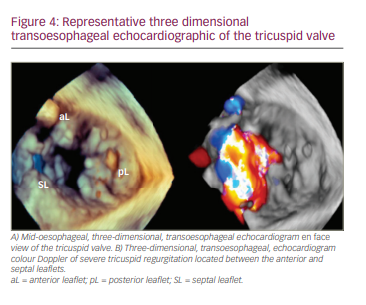
Transoesophageal echocardiography (TOE) can then be used to more accurately assess the complex TV anatomy. Specifically, TOE offers the ability to obtain short-axis gastric plane images of the TV, which allows simultaneous visualization of each leaflet and can readily define specific sites of regurgitation.10 Three-dimensional TOE can further increase the accuracy of TV assessment (Figure 4).14 However, accurate imaging of the tricuspid annulus and surrounding structures remains difficult with any echocardiographic modality, so computed tomography (CT) has become standard prior to transcatheter annuloplasty/replacement or valve-in-ring interventions.14
CT imaging accurately assesses the tricuspid annulus and represents ring geometry. Importantly, it measures tricuspid annulus diameter and tricuspid annulus to right ventricle apex distance, which are paramount in accurately planning replacement devices that interact with the tricuspid annulus and/or TV leaflets.15 Landing-zone geometry is also well defined by CT, and images can be used to identify septal anchor sites, which are required for certain devices.14 CT imaging can also broadly identify impediments to device delivery, including poor venous calibre, calcified papillary muscles and chordae, heavy trabeculation or proximity to easily damaged structures, such as the non-coronary sinus and RCA.11,16 Multiple imaging protocols exist for CT assessment of the TV, and the specifics depend on the choice of device, which is beyond the scope of this review.
While echocardiography is imperative for TV assessment, CT is reserved for replacement/annuloplasty interventions, and magnetic resonance imaging can be used adjunctively in cases where the RV morphology or TR severity are poorly defined.14,17
Transcatheter therapies
Given the high mortality of TV surgery, multiple transcatheter therapies for tricuspid intervention are being investigated.6 The transcatheter strategies can largely be divided into four groups that include edge-to-edge repair, TV replacement, tricuspid annuloplasty and palliative tricuspid therapy.
Edge-to-edge tricuspid valve repair
TriClip
The TriClip™ device (Abbott Cardiovascular, Plymouth, MN, USA) is a transcatheter edge-to-edge repair device that is extremely effective in reducing the severity of valve regurgitation.18,19 The device harnesses the surgical concept of edge-to-edge valve approximation via a 24 Fr transfemoral delivery system. It consists of a 4 mm cobalt–chromium clip and two gripper arms that can be opened and closed independently. The clip is delivered over a regurgitant jet by a steerable catheter, and valve leaflets are clasped, anchoring the clip in place (Figure 5). Given the success of the MitraClip™ (Abbott Cardiovascular, Plymouth, MN, USA), the system was first adopted for use in 3 patients with severe TR in 2015, and feasibility was demonstrated with 100% procedural success rate and no procedural deaths.20
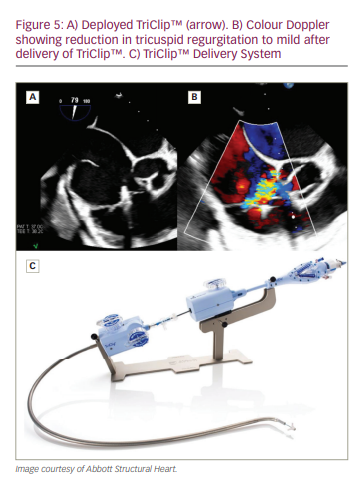
Edge-to-edge TV repair has since become the most commonly performed transcatheter tricuspid intervention worldwide, and 1-year outcomes from the Transcatheter Tricuspid Valve Therapies (TriValve) registry were reported in 2019 in 249 patients who underwent attempted repair with the TriClip™.21,22 Procedural success was defined as clip delivery and reduction in TR to grade ≤2+, which was achieved in 77% of patients, with 1–3 clips delivered per patient. Durable TR reduction and improvement of New York Heart Association (NYHA) functional class to ≤II were observed in 72% and 69% of patients, respectively, at 1 year.22
The TRILUMINATE single-arm study then assessed the safety and efficacy of the TriClip™ system. Results were reported for 6-month and 1-year outcomes.23,24 At 6 months, 71/83 patients (86%) had reduction in TR severity by at least one grade, with 3/83 patients (4%) dying in the study period.23 At 1 year, the reduction in TR severity was maintained in 71% of patients, and there were improvements in NYHA functional class in 83% of patients, in 6-minute walk test (6MWT) by 31 m on average, and in quality-of-life scores by an average of 20 points. All-cause mortality was 7% at 1 year.24
The TRILUMINATE Pivotal trial is on-going and will compare patients randomized to medical therapy versus TriClip™ (TRILUMINATE Pivotal Trial; ClinicalTrials.gov identifier: NCT03904147).25 Given the high technical success and operator familiarity with the MitraClip™ system, edge-to-edge TV repair has set the standard for transcatheter-based repairs.26
PASCAL transcatheter valve repair system
The PASCAL Repair System (Edwards Lifesciences, Irvine, CA, USA) consists of a PASCAL or PASCAL Ace implant, at 10 mm and 6 mm, respectively, with a delivery system. The PASCAL implant consists of a spacer and two clasps that grasp the valve leaflets (Figure 6).27 The clasps can be operated independently and repositioned until final device delivery to allow for staged leaflet capture. The device is delivered transfemorally via a 22 Fr system, and was originally designed for treating mitral regurgitation.27 The PASCAL Ace implant complements the PASCAL platform, and features a smaller spacer that fills the regurgitant orifice, designed to reduce stress on leaflets. Its narrower 6 mm-wide paddle profile allows increased curvature around the spacer for more leaflet surface area capture and a larger neo-coaptation area. The PASCAL Ace implant also allows a staged leaflet capture with independent actuation.28
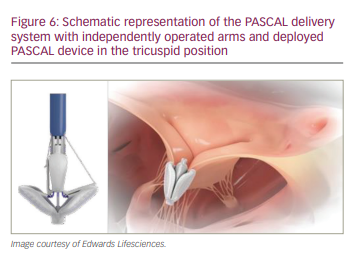
The first TV implantation using the PASCAL Repair System was reported in 2018 in a patient with torrential TR.29 At 30-day follow-up, the patient’s NYHA class reduced from IV to II, there was a 135 m improvement in 6MWT, and an improved quality-of-life score. TTE on follow-up revealed TR reduction to mild.29
After the first case report of the PASCAL Repair System for TR, the first-in-human compassionate-use experience was reported for 28 patients.30 Procedural success was achieved in 24/28 patients (86%) with no procedural deaths. At 30-day follow-up, two patients had died (7.1%); one was due to a presumed cardiac cause and the other due to a heart failure rehospitalization, although with preserved TR reduction prior to death. Of the remaining patients, 88% had NYHA functional class I or II symptoms, TR severity was reduced to ≤2+ in 85% and 6MWT was improved significantly from baseline in 88%. One-year follow-up results demonstrated 93% survival rate and sustained moderate-or-less TR in 86% of patients. Around 90% of patients had NYHA functional class I or II and 6MWT improvement.30
Following the encouraging results from the compassionate-use experience, the CLASP TR early feasibility study (Edwards CLASP TR EFS [CLASP TR EFS]; ClinicalTrials.gov identifier: NCT03745313) reported 30-day outcomes in 2021.31 The study included 34 patients, of whom 29 patients were implanted with the PASCAL Repair System.31 At 30 days, there was no cardiovascular mortality, and TR reduced by at least one grade in 85% of patients and at least two grades in 70%. As with the compassionate-use trial, significant improvements in NYHA class,
6MWT and quality-of-life scores were noted.31
Tricuspid valve replacement
EVOQUE tricuspid valve replacement system
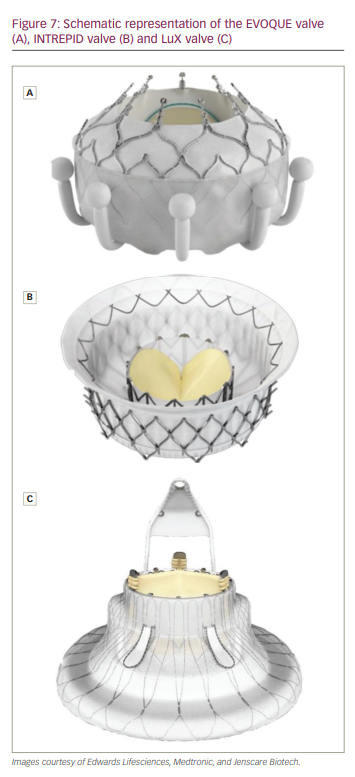
The EVOQUE system (Edwards Lifesciences, Irvine, CA, USA) is a nitinol, self-expanding, bioprosthetic valve composed of bovine tissue, which is delivered transfemorally via a 28 Fr system and anchored within the TV (Figure 7A). The EVOQUE valve comes in multiple sizes (52 mm, 48 mm and 44 mm) to address a broad range of tricuspid pathologies and anatomies.32
The first use was reported in 2020 by Fam et al. in a single patient.33 Procedural success was achieved, and at 6 months the patient had mild TR, NYHA class I symptoms, improved 6MWT (by 200 m) and improved quality-of-life scores.33 The EVOQUE system was used in a first-in-human compassionate-use study in 25 patients.32 Notable outcomes at 30 days included technical success in 23/25 patients (92%) and 0% mortality. TR was reduced to grade ≤2+ in 96%, and NYHA functional class improved to ≤II in 76%.32
The prospective, multicentre TRISCEND study tested the safety and effectiveness of the EVOQUE system in 132 patients.34 At 6 months, there was TR reduction to mild or less in all 43 patients who followed up at that timepoint and NYHA functional class improvement to class I or II in 89%, as well as a significant improvement in quality of life.34 The early success of the EVOQUE system has prompted the TRISCEND II pivotal trial, which plans to enrol over 700 patients to receive the EVOQUE system versus optimal medical therapy (TRISCEND II Pivotal trial;
ClinicalTrials.gov identifier: NCT04482062).35 Successful results could offer options for patients deemed poor candidates for repair, including those with torrential TR.
GATE system
The GATE system (NaviGate Cardiac Structures, Lake Forest, CA, USA) is a nitinol, self-expanding valve consisting of three equine-derived valves. The proximal and distal termini of the nitinol stent are characterized by 12 winglets and 12 graspers, respectively, that are sequentially deployed at fixed angles to radially anchor the device in place.36 The system can be delivered via 42 Fr internal jugular access or transatrially via mini-thoracotomy. Valve sizes range from 36 mm to 52 mm.37
The first-in-human experience was reported in 2017 in 2 patients, both of whom experienced procedural success.38 Since then, 34 patients have undergone implantation on a compassionate-use basis with successful device delivery in 29/34 patients (85%).39 The approach was transatrial in 25 patients and transjugular in 4 patients. At 30 days, 96% of patients had mild TR or less, and 91% of patients had NYHA functional class II or better. Notably, 5/29 cases (17%) required conversion to surgery due to device malposition. Mortality was reported for >30 days in those that had a successfully positioned device, and of these, 5/21 patients (24%) in the transatrial group died while 1/3 patients (33%) in the transjugular group died.39 Given the higher-than-expected mortality, a larger population with longer follow-up period is currently being investigated, and increased attention focused on the technical challenges of delivery.39
INTREPID system
The INTREPID system (Medtronic, Fridley, MN, USA) is a novel, nitinol, self-expanding, bioprosthetic valve composed of bovine tissue that can be delivered by a 35 Fr venous access point.40 The valve is available in three outer frame sizes (43 mm, 46 mm or 50 mm diameter), with a 27 mm inner stent frame.41 The INTREPID system differentiates itself from other transcatheter TV systems in that it does not require leaflet capturing for anchoring, but rather is anchored by deploying a large, fluted, atrial brim on the atrial side of the valve. This brim is flexible and conforms to the atrial shape under which the stiffer ventricular portion sits (Figure 7B).41 Theoretically, this should improve implantation success in those with delicate valve anatomy, and, in one case, has shown to be feasible in the presence of pacing leads.40
The system has been used previously in the mitral position; in 50 patients studied in 2015–2017, procedural success was achieved in 98% of patients, with 7 deaths in the first 30 days, most of which were secondary to complications of the transapical delivery approach. At 6 months, there were 4 additional deaths, and the 1-year survival rate was 77%.41 Thus far, a small case series using the device in the tricuspid position for 3 patients was reported in 2020, where successful delivery was achieved in all cases.40 An early feasibility trial using the system for TV disease in 15 patients is underway in the USA without any reported results to date (TTVR early feasibility study; ClinicalTrials.gov identifier: NCT04433065).42
LuX-Valve
The LuX-Valve (Jenscare Biotechnology, Zhejiang, China) is a tri-leaflet, self-expanding nitinol valve composed of bovine tissue. The valve has an atrial disc and septal anchor or ‘tongue’ with two graspers for leaflet fixation (Figure 7C). It can be delivered via mini-thoracotomy with a 32 Fr catheter and is available in 50 mm, 60 mm and 70 mm discs, with 26 mm and 28 mm valves.43,44
The first-in-human use was published in 2020 and included 12 patients.43 Procedural success was achieved in all patients, with one death in the 30 days post-procedure. Follow-up at 30 days showed mild TR or less by TTE in 10/11 (91%) patients, improved 6MWT by an average of 177 m, and reduction of NYHA class III/IV symptoms to NYHA class II symptoms in 55% of patients.43 A subsequent compassionate-use trial of 35 patients reported 100% procedural success and 30-day mortality of 6%. Improvements in 6MWT and NYHA class were observed.44 Of note, similar to the INTREPID system, the LuX-Valve has been successfully implanted in at least 5 patients with pre-existing pacemaker leads without disruption.43
Tricuspid annuloplasty
Transcatheter tricuspid annuloplasty devices are modelled after surgical annuloplasty repair procedures and were created to address the primary pathophysiology of patients with TR secondary to annular dilation. The two broad categories of annuloplasty devices are suture-based devices and ring or non-suture devices.45 Extrapolating surgical data, both approaches should theoretically have similar outcomes, but the suture annuloplasty devices are more widely used in current practice and have more data supporting their use.46
Suture-based devices and techniques
TriCinch™
The TriCinch™ (4Tech Cardio Ltd, Galway, Ireland) reproduces the surgical Kay procedure by cinching the anteroposterior commissure, thereby reducing the septolateral dimensions of the tricuspid annulus. An 18 Fr steerable delivery system is advanced through a 24 Fr transfemoral sheath to access the TV. A stainless-steel corkscrew anchor is fixed at the anteroposterior commissure and connected to a nitinol stent through a Dacron band that is deployed in the subhepatic region of the inferior vena cava (IVC) to maintain tension.47
The first-generation TriCinch™ device was first studied in 24 patients in the PREVENT trial (Early feasibility study of the percutaneous 4Tech TriCinch Coil Tricuspid Valve Repair System; ClinicalTrials.gov Identifier: NCT03632967).47 The procedural success rate was 85%, with the reasons for failure being haemopericardium and late detachment of the anchor. Overall, 94% of patients had significantly reduced TR, and 6-month follow-up data showed improved quality of life in most patients.48
As a result of the high rates of haemopericardium, a second-generation TriCinch™ Coil System was developed to replace the corkscrew system previously described. The anchor is delivered in the pericardial space, rather than the annulus, providing increased surface area, tension and stability.47 The first-in-human case was performed with no complications and saw improved severity of TR and quality of life at 30 days.49
Trialign™
The Trialign™ device (Mitralign, Tewksbury, MA, USA) replicates the modified Kay procedure by obliterating the posterior leaflet. Transjugular access is used to place two pledgets sequentially at the posteroseptal and anteroposterior commissures, which are then cinched together to reduce annular dimensions. A second pair of pledgets can be placed if further reduction in annular dimensions is required.16,50
The safety and feasibility of the Trialign™ device was first studied in 14 patients for compassionate use. It showed a 42% reduction in vena contracta width, a 51% reduction in effective regurgitant orifice area (EROA) and a 34% reduction in annular area, with only one procedural complication, which was an arrhythmia.51 The SCOUT I trial (Early feasibility of the Mitralign Percutaneous Tricuspid Valve Annuloplasty System [PTVAS] also known as TriAlign™. [SCOUT]; ClinicalTrials.gov identifier: NCT02574650) enrolled 15 patients and found a 93% procedural success rate, with 80% technical success rate at 30 days.52 One patient required RCA stenting due to extrinsic compression and 3 patients had single-pledget dehiscence without the need for reintervention. At 30-day follow-up, there were no major adverse events, and a significant reduction in tricuspid annular area and EROA was noted, with improvement in NYHA functional class.52 These benefits were durable at 1-year follow-up, with only one patient requiring reintervention.53 Given the encouraging results, the SCOUT II trial is an on-going prospective, single-arm, multicentre, open-label study that will enrol up to 60 patients from up to 15 sites in Europe and the USA, with the primary endpoint being 30-day all-cause mortality (Safety and performance of the Trialign Percutaneous Tricuspid Valve Annuloplasty System [PTVAS] [SCOUT-II]; ClinicalTrials.gov identifier: NCT03225612).54
Pledget-assisted suture tricuspid annuloplasty
The pledget-assisted suture tricuspid annuloplasty (PASTA) technique is based on the Hetzer’s double-orifice suture technique and creates a double-orifice TV, as two sutures and one pledget are sequentially placed at the mid-anterior and posteroseptal annulus.55 The first-in-human results were recently presented, showing feasibility.56
Non-suture devices
Cardioband
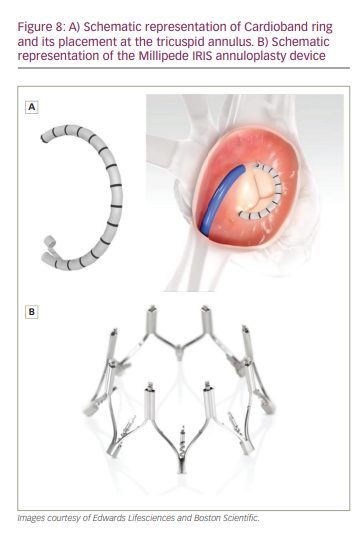
The Cardioband tricuspid system (Edwards Lifesciences, Irvine, CA, USA) is an adjustable, polyester fabric band that is inserted through a transfemoral 24 Fr sheath and deployed on the atrial side of the anterior and posterior annulus (Figure 8A). The size can then be adjusted as needed to reduce the tricuspid annular diameter.16,57
This device received the CE mark of approval in 2018, based on the results of the TRI-REPAIR study, which enrolled 30 patients with at least moderate functional TR.58 Technical success was 100%, and periprocedural events included 2 deaths due to RV failure and bleeding unrelated to the device, 1 stroke and 3 major bleeding events.59–61 Notable results from the 6-month follow-up included an average reduction in annular septolateral diameter of 9%, a reduction in EROA of 50% and a reduction in vena contracta width of 28%, compared with baseline. There was at least one NYHA functional class improvement in 76% of patients, with 88% in NYHA functional class I or II.61 These results were sustained at the 2-year follow-up, with a reduction in septolateral diameter of 16%, a TR reduction to moderate or less in 72%, and NYHA functional class I or II in 82% of patients. Survival rates at 1 and 2 years were 83% and 73%, respectively.62
European post-market clinical outcomes of the TriBAND study demonstrated favourable 30-day results in 61 patients, with a 20% reduction in septolateral diameter. Around 85% of patients had at least a one grade reduction in TR, and 74% of patients improved NYHA functional class to class I or II.63
Similar results were recently demonstrated with the US Edwards Cardioband TV reconstruction system early feasibility study (ClinicalTrials.gov identifier: NCT03382457), which enrolled 30 patients.64 Device success was 93.3%, and placement of the Cardioband tricuspid system resulted in a 13% reduction in annular septolateral diameter, 35% reduction in EROA and 39% reduction in vena contracta width at 30 days. As well, 85% of patients had at least a one-grade reduction in TR. In addition, 75% of patients were NYHA functional class I or II at 30 days. Mortality after 30 days was 0%, and the most common procedural major adverse event was severe bleeding, which occurred in 7 patients.64
Minimally invasive annuloplasty
The minimally invasive annuloplasty (Micro Interventional Devices, Newtown, PA, USA) is a suture-free system that is composed of a thermoplastic elastomer (MyoLast™; Micro Interventional Devices) and low-mass polymeric, compliant anchors (PolyCor™; Micro Interventional Devices) that permits annular reduction via a 16-Fr delivery system in a 270° partial ring pattern.16,45,57,65 The safety and feasibility of this device will be studied in the STTAR trial (Study of transcatheter tricsupid annular repair; ClinicalTrials.gov identifier: NCT03692598), which will enrol 60 patients.66 Early results presented in 2020 showed a significant reduction in valve area, TR grade, EROA, vena contracta width and valve diameter at 30 days in the 24 patients studied.65 A further 13 patients had follow-up at 6 months, with echocardiographic improvement.65 No mortality data were reported.
Millipede IRIS
The Millipede IRIS System (Boston Scientific, Marlborough, MA, USA) is a complete, rigid, adjustable ring that mimics a surgical annuloplasty ring (Figure 8B). It uses a 27 Fr delivery system via transfemoral or trans-septal access and is placed in supra-annular position to reduce annular size. This system is unique in that the delivery system includes an integrated, 8-Fr, intracardiac, echocardiographic imaging catheter intended to facilitate procedural execution. The device can be retrieved and adjusted prior to final deployment, and also preserves native anatomy, permitting future TV interventions.67 The device has been implanted in 2 patients, and at 12 months, the tricuspid annulus diameter had reduced by 40%.67 A dedicated tricuspid delivery system is currently being designed.68
Transatrial intrapericardial tricuspid annuloplasty
The transatrial, intrapericardial, tricuspid annuloplasty (TRAIPTA) is a fully retrievable, transpericardial annuloplasty device under preclinical investigation. A right atrial appendage puncture is made into the pericardium through transfemoral access, and an implant is placed along the AV groove, externally compressing the tricuspid annulus from the pericardial space. The device has been successfully implanted in animal models, and a human-specific iteration is currently being developed.69,70
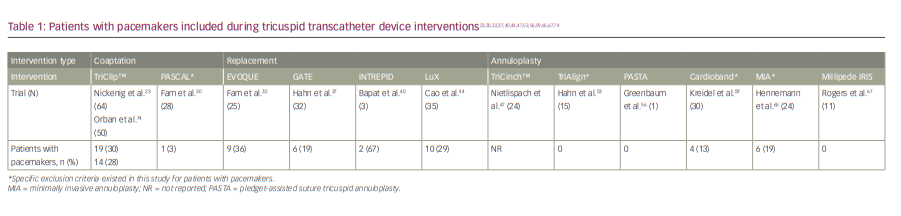
Presence of a pacemaker lead
Worldwide, there are over 700,000 pacemakers or internal cardioverter defibrillators implanted annually.71,72 Additionally, the presence of a pacemaker lead traversing the TV may result in TV leaflet restriction and contribute to the presence or severity of TR.73 Fortunately, several transcatheter therapies have been investigated in this population and have shown feasibility (Table 1).23,30,32,37,40,44,47,53,56,59,65,67,74 Notably, valve repair or annuloplasty can be more challenging in this population, as the pacemaker leads must be avoided when grasping valve leaflets for coaptation or deploying annuloplasty anchors.75 Conversely, each current iteration of transcatheter valve replacements has shown success in those with a pacemaker.32,37,40,44
Tricuspid valve-in-valve and valve-in-ring
The longevity of surgical TV prostheses is lower than other prosthetic valve devices.76,77 The choice to use mechanical valves in the tricuspid position when repair is not feasible is less common than in left-sided valve procedures, due to their higher risk of valve thrombosis in the low-pressure system.78 The subsequent use of bioprosthetic valves can then theoretically increase the rate of time-related valve failure with either regurgitation or stenosis, and a repeat surgical procedure is often not feasible in patients with multiple comorbid conditions.78 The possibility of transcatheter valve-in-valve (TVIV) and transcatheter-valve-in-ring (TVIR) thus becomes an attractive option.
TVIV and TVIR procedures have been previously well described and will be briefly summarized here.79,80 Access is typically transfemoral, but transjugular access can be used in cases with extremely horizontal valves. The tricuspid prosthesis is typically crossed with a catheter and shaped wire, but steerable sheaths, such as the Agilis™ NxT (Abbott Cardiovascular, Plymouth, MN, USA) or Dexterity™ (Spirus, Bridgewater, MA, USA) can be used with a Glidewire® (Terumo Interventional Systems, Ann Arbor, MI, USA) in challenging cases. Once the wire has successfully crossed the TV, it is stabilized by parking it in the left pulmonary artery, forming a coaxial rail for valve delivery, or via a RV loop, which is often the preferred method if the right ventricle is dilated. The transcatheter heart valve is delivered on this wire and typically positioned and deployed using fluoroscopic guidance (Figure 9). Temporary pacing for stabilization may be required in cases of excessive cardiac motion or when performing a TVIR.79,80
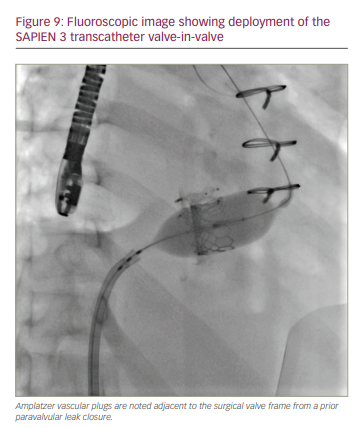
The ultimate success of the procedure requires correct sizing and placement of the valve during deployment. This depends on careful and meticulous pre-procedural planning with CT imaging and by reviewing prior surgical records to better understand the size of the prosthetic device.79 There are currently two available valves that can be used: the Melody™ valve (Medtronic, Minneapolis, MN, USA) and the SAPIEN 3 valve (Edwards Lifesciences, Irvine, CA, USA).79
Additional knowledge is required for TVIR, which includes determining if the prior implant is flexible, semi-rigid or rigid, and if it is complete or incomplete. Some general principles are as follows: incomplete bands or rings make it more difficult to properly anchor the valve; flexible rings and bands may expand during deployment, which can lead to under-sizing; incomplete flexible rings and bands have the greatest potential for embolization; and rigid and semi-rigid bands and rings have the greatest risk of deformation or peri-leak.81 Peri-leak is more common in TVIR compared with TVIV, as most implanted tricuspid annulus rings are incomplete rigid rings, which can limit full circumferential expansion of a transcatheter-delivered valve.79
TVIR is feasible in patients with pre-existing pacemaker leads, and jailing the device lead is generally safe and well tolerated.82 However, if the patient depends on the lead for pacing, lead impedance and thresholds must be carefully monitored immediately after device deployment to ensure proper functioning.79
The first successful TVIV was performed in 2011 using a 23 mm SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA).83 Since then, additional case reports and registries have demonstrated the success of this procedure.84,85 The largest registry of TVIV demonstrated a procedural success rate of 99%, with 62% of patients receiving a Melody™ valve, and the remainder a SAPIEN valve.85 Minimal procedural complications were noted. At a median follow-up of 13.3 months, 22 patients (14%) died, 5 of whom died within 30 days. At follow-up, 77% of patients were NYHA functional class I or II, compared with only 29% prior to intervention. Importantly, outcomes did not differ based on the type of valve implanted.85
Meanwhile, the largest registry of TVIR demonstrated a procedural success rate of 91%, although there were only 22 patients in the registry.86 Of them, 20 patients underwent implantation, with 17/20 patients (85%) receiving a SAPIEN valve and 3/20 patients (15%) receiving a Melody™ valve. No procedure-related deaths were reported; however, there was 1 case of valve embolization and 1 case of severe paravalvular leak that required an additional immediate TVIR. At a median follow-up of 12 months, 1 patient had died and 2 had undergone repeat TVIR implantation. Fifteen patients (75%) had evidence of paravalvular leak by TTE at follow-up, but most paravalvular leak were trivial to mild in severity.86
McElhinney et al. published mid-term valve-related outcomes in 306 patients that underwent either TVIV or TVIR.87 The cumulative 3-year incidences of death, reintervention or valve-related adverse outcomes, which included endocarditis, thrombosis or significant dysfunction, were 17%, 12% and 8%, respectively.87 While long-term data on efficacy are still limited, TVIV and TVIR remain promising procedures for patients who, often, have no other viable corrective options.
Palliative tricuspid therapy
Outside of altering native valve anatomy, heterotopic caval valve implantation (CAVI) can be done to reduce the degree of regurgitation entering the caval system, thus decreasing the systemic congestion that leads to hepatic venous congestion, renal dysfunction and peripheral oedema.88,89
There are two different devices that have been studied for CAVI: a balloon-expandable 29 mm SAPIEN valve that is currently used for transcatheter aortic valve replacement, and a dedicated,
self-expandable TricValve® (P+F Products + Features, Wessling, Germany). The TricValve® was the first type used clinically, initially implanted in 2011, and resulted in reduced caval pressure and improved functional status at the 8-week follow-up.89,90 It consists of a bovine pericardial skirt and leaflet apparatus that is surrounded by a nitinol frame. The superior vena cava (SVC) valve is available in 25 mm (used for 22–31 mm SVC diameters) and 29 mm (used for 27–34 mm SVC diameters) and is designed with a long skirt to prevent paravalvular leak. In contrast, the IVC valve is available in 31 mm (used for 24–31 mm IVC diameters) or 35 mm (used for 28–35 mm IVC diameters) and is designed with a short skirt to prevent hepatic occlusion.91
The safety and feasibility of the balloon-expandable 29 mm SAPIEN valve was first demonstrated in 2013 in 3 patients, with 2 of the patients receiving IVC valve placement only and the other receiving bicaval valves.92 There were no adverse outcomes at the 30-day follow-up and no evidence of paravalvular regurgitation, with all patients maintaining reduced caval pressures and improved NYHA functional class.92
Subsequently, multicentre observational studies have since demonstrated the safety and efficacy of CAVI for patients with severe TR. Lauten et al. examined the role of CAVI in 25 patients between 2010 and 2017.93 Most patients (76%) had IVC implantation only, with the balloon-expandable 29 mm SAPIEN valve being the valve most selected (84%). Procedural success was achieved in 96% of patients, with device embolization requiring surgical retrieval in 1 patient. One-year mortality was high at 63% and was attributed to multi-comorbid conditions. There was a significant improvement in NYHA functional class and reduction in right atrial and IVC pressure, with no significant change in cardiac index.93 The first CAVI registry in the USA was reported in 2020 using the SAPIEN valve in the IVC position.94 The results were notable for 100% procedural success rate, with 73% of the patients improving at least one NYHA functional class at median 30-day follow-up (range 14–64 days).94
There are no head-to-head data comparing the effectiveness or outcomes of the SAPIEN valve to the TricValve®; most published cases use the SAPIEN valve due to its commercial availability. Caval dilation remains the main limitation in both devices, as the IVC may dilate up to 35 mm in diameter, while the SVC may dilate up to 40 mm in chronic severe TR due to volume overload.16 One potential solution is surgical downsizing of the IVC, but most patients being considered for this therapy already represent prohibitive surgical risks.95 The tapered dilatation of the SVC makes placement of the SAPIEN valve challenging, which is why TricValve® is preferred in this location.93 It is currently believed that bicaval CAVI is not superior to implantation in the IVC alone, although this still requires further analysis.96
Finally, while the registries presented above have shown excellent procedural success and safety in the short term, device embolization remains a potential complication of this procedure.97
The TRICAVAL trial (Treatment of severe secondary tricuspid regurgitation in patients with advance heart failure with caval vein implantation of the Edwards Sapien XT valve; ClinicalTrial.gov identifier: NCT02387697), a randomized controlled trial of CAVI with the SAPIEN valve versus optimal medical therapy, had to be terminated early due to 28.5% of patients having device embolization requiring open-surgical retrieval.97 On-going clinical trials, including the HOVER trial (Heterotopic implantation of the Edwards-Sapien transcatheter aortic valve in the inferior vena cava for the treatment of severe tricuspid regurgitation; ClinicalTrials.gov identifier: NCT02339974) with the SAPIEN valve and the TRICUS STUDY Euro (TRICUS STUDY Euro – safety and efficacy of the TricValve® device; ClinicalTrials.gov identifier: NCT04141137) with the TricValve® aim to shed further light on the safety and efficacy of CAVI.98,99
Conclusions
Severe TV pathology remains a highly morbid condition with poorly defined criteria for intervention, and high surgical mortality.1,5,6 The recent increase in transcatheter-based TV therapy systems has given practitioners additional options for treating TV disease, and early results from compassionate-use studies have generally been encouraging. As experience with these systems becomes more widespread and more patients are studied, transcatheter tricuspid therapies could offer alternatives for treating TV disease and inform more pragmatic
guidelines on the timing and methods of intervention.







