Heart failure (HF) is a highly prevalent and morbid disease in the USA, imposing a significant burden on patients, hospitals and the health system. The prevalence of HF continues to increase, with over 650,000 new HF cases diagnosed annually.1 There are over 6.2 million people with HF in the USA,2 with an expected increase to 8 million by 2030.3 Despite recent additions to the pharmacologic armamentarium available to treat HF, symptomatic HF remains challenging to manage, with poor outcomes and reduced quality of life for patients and caregivers.4–8 HF is a chronic and progressive disease, characterized by acute exacerbations often necessitating hospitalization. The result is over 1 million HF hospitalizations costing $30 billion annually, with costs expected to double in the next decade.9,10 Re-admissions are common, with up to 25% of patients re-admitted within 30 days post-discharge, and 50% of patients re-admitted within 6 months.10 These re-admissions are particularly costly in the context of health policies, which penalize hospitals with higher-than-predicted re-admission rates.11
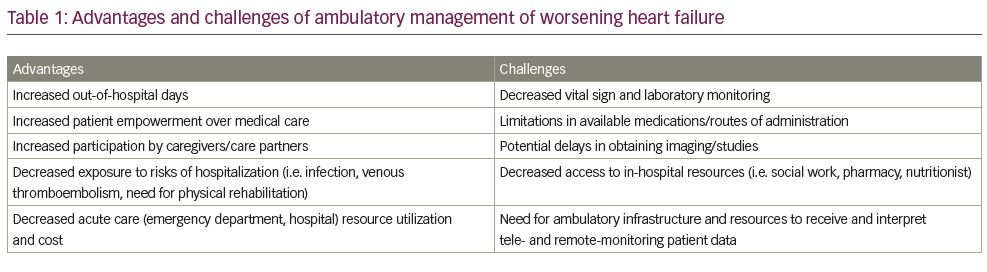
Strategies that promote improved transition of care from inpatient to ambulatory settings may help decrease the economic toll of HF on the healthcare system, while alleviating patient burden.12 Potential benefits include maximizing patient time at home, preventing delays in care associated with emergency department visits and avoiding potential complications of hospitalizations (Table 1).13 Here we review the current state of outpatient HF management, including defining worsening HF, describing patient monitoring and treatment strategies and identifying opportunities for ambulatory HF care delivery. Further, we outline future directions for the ambulatory management of this expanding patient population.
Defining worsening heart failure
The progressive course of HF is characterized by a slow clinical decline over years with episodic exacerbations, described as ‘worsening heart failure’ (WHF).14–16 HF symptoms – including lower extremity oedema, dyspnoea at rest and with exertion, orthopnoea, paroxysmal nocturnal dyspnoea and abdominal distention – are largely attributable to congestion.17 Symptoms of congestion are associated with reduced quality of life in HF and are frequent reasons patients present for acute care.17–19 Historically, WHF has required hospitalization for treatment with intravenous (IV) loop diuretics.20 WHF is increasingly recognized as a clinical entity that can be treated with escalation of therapy in the ambulatory setting, possibly avoiding hospitalization.14 WHF is in fact now included as a clinical endpoint, separate from mortality or HF hospitalization, in clinical trials.16 Importantly, while hospitalization confers a significant increase in mortality, recent data suggest that ambulatory patients with WHF also suffer a poor prognosis.21–24 Thus, strategies for the prevention, detection and prompt treatment of WHF episodes can aid HF management in the outpatient setting.
Detection of worsening heart failure through patient monitoring
Self-care practices
Effective monitoring of the patient’s clinical status is central to timely outpatient HF care. The burden of daily evaluation for signs and symptoms of WHF falls largely on patients and caregivers.25 Symptom monitoring, in addition to adherence to medications and preventative behaviours (such as dietary fluid and sodium restriction), comprise the foundation of HF self-care maintenance. Patients who are able to engage in self-care management have better outcomes. Among 195 patients with HF, Lee et al. demonstrated that patients with above average HF self-care management had a statistically significant 56% risk reduction in all-cause mortality, hospitalization or emergency room admission.26 Unfortunately, other studies have shown that HF self-care behaviours are performed infrequently and symptoms are often misinterpreted as unrelated to HF.27,28
Adjuncts to patient self-care include structured telephone support and home visits, which are often employed after hospital discharge in efforts to prevent re-admission.29 Structured telephone calls provide opportunities for monitoring and education through regular contact. Home-visiting programmes allow clinicians to meet patients in their home environment and provide reinforcement of self-care practices, physical exams and medication reconciliation. These practices were recently evaluated in a meta-analysis of 47 randomized controlled trials, including 13 structured telephone support and 14 home-visiting programme studies.29 Home visits significantly reduced all-cause readmission (relative risk [RR] 0.75; 95% confidence interval [CI] 0.68–0.86, number needed to treat [NNT] 9) and HF-specific re-admission (RR 0.51; 95% CI 0.31–0.82; NNT 7) over 3–6 months. However, structured telephone support did not significantly reduce the risk of all-cause re-admission or HF-specific re-admission. These findings were demonstrated again in a more recent analysis by Van Spall and colleagues, who also found that home visits reduced healthcare costs compared with usual care.30
Remote monitoring
Remote monitoring involves the use of handheld, wearable or implantable devices to capture and transmit clinical data to a healthcare provider for purposes of monitoring and providing care, often in conjunction with telephone or video consultations.11 Through regular collection of physiologic data, clinicians may be able to identify early signs of decompensation and intervene before hospitalization becomes necessary.31 The past two decades have seen rapid advancements in HF telemonitoring devices, with many more on the horizon.32 One strategy incorporates implantable cardiac device-based diagnostics, such as intrathoracic impedance, heart rhythm and heart rate variability, to identify patients at high risk of hospitalization.33–35 In the MultiSENSE (Multisensor Chronic Evaluation in Ambulatory Heart Failure Patients) trial, the multisensor-based algorithm HeartLogic (Boston Scientific Corporation, Marlborough, MA, USA) was developed and validated in a study of 900 patients.36 The algorithm was found to have sensitivity of 70% (95% CI 55.4–82.1%) and alerted a median of 34 days prior to a HF event (interquartile range [IQR] 19.0–66.3 days).
Implantable wireless haemodynamic monitors are an alternative to device-based algorithms. One such device is the CardioMEMS HF System (CardioMEMS, Inc., Atlanta, GA, USA), a sensor that directly measures pulmonary arterial pressures and transmits data wirelessly. The prospective, single-blinde CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients) trial randomized 550 patients to management with a wireless haemodynamic monitoring system, incorporating daily pulmonary artery pressure measurements (treatment) or control.37 Over 6 months, there was a 28% risk reduction in HF-related hospitalization in the treatment group (hazard ratio [HR] 0.72; 95% CI 0.60–0.85; p=0.0002). A post-hoc analysis of patients with left ventricular ejection fraction receiving HF guideline-directed medical therapy also demonstrated a 47% decrease in mortality (HR 0.63; 95% CI 0.41–0.96; p=0.0293).38 A recent meta-analysis of five trials of implantable wireless haemodynamic monitoring systems demonstrated similar results, finding a 38% reduction in HF events when compared with standard care (HR 0.62; 95% CI 0.50–0.78; p<0.001).39 These data prompted the European Society of Cardiology 2016 HF guidelines to include the use of implantable pulmonary artery pressure monitoring in patients with a previous HF hospitalization as a class IIb recommendation to reduce the risk of recurrent hospitalization.40
Despite several studies failing to demonstrate benefit, meta-analyses have suggested that telemonitoring interventions can decrease HF hospitalizations and improve mortality.41–44 A more recent analysis of multiple systematic reviews by Kitsiou et al. further delineated the individual impact of four categories of telemonitoring interventions in an exploratory post-hoc analysis: automated device-based telemonitoring, mobile telemonitoring, interactive voice response systems, and video-conferencing with vital signs monitoring.31 Of these approaches, automated device-based telemonitoring demonstrated the most significant benefit, with a 35% reduction in all-cause mortality (RR 0.65; 95% CI 0.54–0.79; p<0.001) and 23% RR reduction in HF-related hospitalizations (RR 0.77; 95% CI 0.64–0.91; p=0.003). Interactive voice response systems, involving the manual input of data by the patient into a telephone keypad in response to automated voice prompts, were not shown to reduce mortality or hospitalizations. However, Kitsiou et al. note that prior analyses have evaluated multiple types of telemonitoring approaches as one standardized intervention, potentially obscuring the differences in efficacy of each approach. The lack of high-quality evidence to support or dispute the benefits of telemonitoring suggests that more data are needed to guide telemonitoring practice in HF.31,45
Ambulatory treatment of worsening heart failure
Identification of WHF requires prompt intervention to decrease congestion and circumvent hospitalization. Loop diuretics, such as furosemide, are a cornerstone in the treatment of both acute and chronic heart failure. Loop diuretics block sodium reabsorption in nephrons, facilitating natriuresis.46,47 Bioavailability varies by agent and administration route.47 In WHF, diuretic absorption and effect can be reduced due to gut wall oedema or decreased abdominal blood flow.48 Bumetanide and torsemide (torasemide) both offer higher, more reliable oral absorption (80–100% bioavailable) and may be options for patients who do not respond to oral furosemide.46,47 An alternative approach is the addition of a thiazide diuretic, such as metolazone. These drugs inhibit the compensatory sodium avidity seen in the distal nephron in response to loop diuretic use, thus augmenting natriuresis by a complementary mechanism.49 With guidance from the care team, patients may be able to titrate diuretics and trial these approaches at home to prevent WHF.
Despite these strategies, patients frequently require IV diuretics for decongestion.50 Historically, treatment with IV diuretics has necessitated inpatient admission; however, health systems are increasingly mobilizing ambulatory HF clinics to offer outpatient IV diuretic administration. These integrated disease management clinics, or same-day access clinics, are staffed with multidisciplinary teams, which may include physicians, advanced practice providers, nurses, pharmacists, dieticians and social workers.11 They are equipped to provide transitional care following hospital discharge, including diuretic titration, guideline-directed medical therapy optimization and HF education. Same-day access clinics can serve an invaluable role in the triage and treatment of WHF, including the administration of IV diuretics. The feasibility and safety of outpatient IV diuretics have been described in several single-centre studies.51–54 Additionally, outpatient IV diuretics may play a role in the management of specific at-risk HF populations, such as those with restrictive cardiomyopathy, with a recent study of ambulatory IV diuresis in patients with cardiac amyloidosis demonstrating a decrease in the number of acute care visits and hospital days.55 An alternative strategy to IV outpatient diuresis on an as-needed basis may be the use of intermittent empirical diuretics, which could also be facilitated through an HF clinic and has shown potential benefit in case reports.56
While the outpatient administration of IV diuretics offers a potentially effective, cost-saving route for the treatment of WHF, this strategy is not without barriers.53,57 Patients are still required to come to the clinic, which may be challenging for symptomatic or immobile patients, or those living in remote areas. Additionally, not all outpatient clinics are equipped to accommodate IV diuretic administration. Medication administration requires placement of an IV line by a skilled nurse or technician, and may be associated with complications such as infection and thrombosis (though rare).10
Future directions
Role of telemedicine and patient monitoring
As noted above, patients with HF may have several barriers to in-person care, and worsening symptoms that might otherwise be managed in the clinic may instead result in calls to emergency medical services. The recent worldwide COVID-19 pandemic has additionally necessitated changes across healthcare systems, drastically reducing ambulatory clinic capacity in favour of virtual visits.58 The effects of social isolation, physical inactivity, emotional stress and dietary changes during the pandemic on patients with HF have been further compounded by patients’ hesitation to seek care and risk exposure.59–61 Recognizing the potential role for telemedicine in the care of patients with HF, the Heart Failure Society of America recently issued an expert consensus statement on the use of telehealth in HF.58 These guidelines, in conjunction with widespread amendments to telehealth-related reimbursement policies across major insurance carriers, have fuelled an interest in developing effective practices for telehealth. Potential benefits include expanding access to timely clinical care, allowing increased caregiver involvement, facilitating clinician interaction with the patient’s home environment and, currently key, reducing potential exposures to COVID-19. However, challenges remain, including limitations in patients’ ability to check vital signs and weight, the inability to perform a comprehensive physical exam, technical difficulties with video conferencing and disparities in access to technology. Furthermore, patients evaluated via a virtual visit may still require in-person assessment or treatment.
Early experience with transitioning to a telemedicine-based strategy has highlighted the importance of empowering patients to use self-management strategies and remote monitoring to enhance virtual visits.62 For example, the early months of the pandemic brought a notable increase in the use of CardioMEMS in Europe, the Middle East and Africa, associated with a decrease in HF hospitalizations.63 Centres in the USA have also reported a reduction in HF hospitalizations among patients monitored by implantable haemodynamic monitoring devices during this period, as well as an increase in clinician–patient interactions related to HF management.64,65 Importantly, it is imperative to retain in-person access to clinics for the purpose of triage and treatment of acutely decompensated patients.62
At-home treatment of worsening heart failure
With notable advancements in remote monitoring and management, the next frontier in HF care is at-home treatment of WHF. Subcutaneous (SC) injections of furosemide present an effective alternative to IV formulations.66–68 Previously, the SC use of commercially available furosemide formulations was impeded by their high alkaline pH, which caused irritation at the injection site.61 The development of a novel, buffered furosemide formulation (scFurosemide; scPharmaceuticals, Inc., Burlington, MA, USA) with a pH of 7.4 helped to alleviate injection site complications.68 Initial studies of this agent, delivered in a biphasic dosing profile via an external infusion pump (30 mg over 60 minutes followed by 12.5 mg/h for 4 hours), demonstrated that it was well-tolerated and effective when compared with the IV formulation.68 Therapeutic furosemide levels were observed as early as 30 minutes after SC infusion initiation and resulted in diuresis and natriuresis similar to that produced by the IV route.68
These studies were followed by a randomized, phase II trial of 41 patients presenting to an ambulatory HF clinic with WHF.69 In the first 6-hour interval after drug administration, patients in the IV and SC groups had similar median urine output (IV 1,325 mL [IQR 1,075–1,350 mL]; SC 1,350 mL [IQR 900–1,900 mL]) and weight loss (IV -1.5 ± 1.1 kg; SC -1.5 ± 1.2 kg). One patient in the SC group experienced mild hypokalaemia; no other adverse events were reported. SC furosemide formulations have also been shown to be of value for patients with HF in palliative settings.70,71
As experience with outpatient use of SC furosemide increases, there is interest in ultimately gaining approval for an SC formulation that can be administered at home.72 Several studies are ongoing or planned in the near-term to assess the safety and efficacy of wearable furosemide drug-delivery systems.73–75 For example, FUROSCIX® (scPharmaceuticals, Inc., Burlington, MA, USA) is pre-programmed to deliver 80 mg of SC furosemide to the patient through an adhesive pump. Patients presenting with WHF both to ambulatory and acute care settings will be treated with these SC furosemide formulations, with outcomes including cost of care, hospitalization, and patient-reported outcomes, such as quality of life.
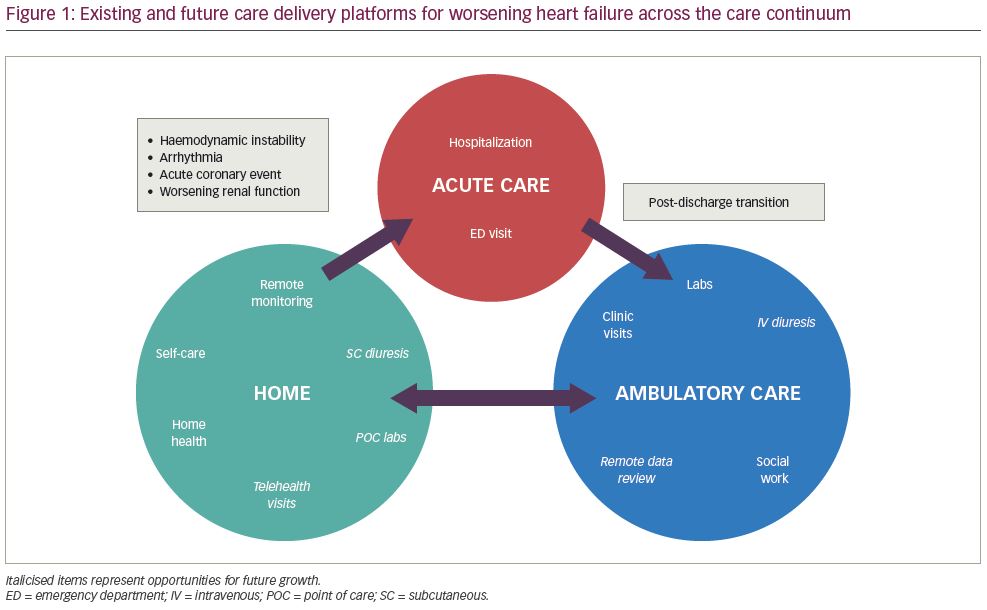
With the use of remote monitoring and televisits to adjust medical therapies and the potential for more potent at-home diuresis, naturally point-of-care mechanisms to monitor electrolytes, such as potassium, are needed. The i-STAT (Abbott Laboratories, Chicago, IL, USA) is one such device that has been investigated for use in home visits.76 One could imagine a future workflow that includes the remote incorporation of haemodynamic data, symptoms and laboratories, which then prompts a clinician-initiated ‘prescription’ for WHF that may be available for pick up or delivery to a patient’s home (Figure 1).
Lastly, in those patients who develop diuretic resistance, for example in the setting of progressive renal dysfunction requiring renal replacement therapy, peritoneal dialysis may be considered as an option that allows patients to remain at home. Several small observational studies have shown that peritoneal dialysis may have a role in the treatment of WHF in patients with end-stage or diuretic-refractory HF with or without end-stage renal disease, decreasing hospitalizations and improving New York Heart Association class.77,78 Peritoneal dialysis may offer ultrafiltration that can relieve congestion without significant fluid shifts, and, importantly, can be managed at home by motivated patients and caregivers.
Conclusion
Heart failure is an increasing health and economic challenge in the USA. Alleviating the toll on patients and their caregivers, as well as decreasing the cost burden on the healthcare system, depends significantly on the ability to transition care from the hospital to the outpatient clinic or, increasingly, to home. Multiple recent innovations in HF management may work to streamline treatment in the ambulatory or home setting and help to provide effective, cost-saving, patient-centred care for patients with HF.