Heart failure (HF) is an increasingly prevalent clinical syndrome resulting from a variety of disease processes impeding the heart’s ability to effectively circulate blood. With advances in care and a steadily growing and aging population, HF management is becoming a foremost priority in developed nations like the USA, where HF is expected to affect more than 3% of the overall population or 8.5 million people by 2030.1 This increase in population burden directly translates into patient morbidity and mortality, as well as the significant economic costs of delivering healthcare, projected to be approximately $70 billion for the USA by 2030.2
The syndrome of HF was first described by several ancient civilizations. A greater understanding of HF has steadily developed over the centuries, and in the early 20th century, modern therapeutics in the form of diuretic medications established a treatment approach for HF.3 Guideline-directed medical therapy (GDMT), medications proven in clinical trials to reduce morbidity and mortality, started in 1986 when the V-HeFT trial demonstrated a mortality benefit with the use of isosorbide dinitrate and hydralazine compared with prazosin and placebo.4 Shortly afterwards in 1987, angiotensin-converting enzyme inhibitors (ACE-I), specifically enalapril, presented mortality benefits in the CONSENSUS trial.5
Subsequent GDMT focused on mortality benefits derived from neuro-hormonal blockade in the form of beta blockers; ACE-I, angiotensin-2-receptor blockers (ARB), and angiotensin receptor-neprilysin inhibitors (ARNI); and mineralocorticoid receptor antagonists (MRA). Additionally, devices such as internal cardioverter defibrillators and biventricular pacemakers have been added to the arsenal of tools to reduce morbidity and mortality. However, the burden of HF remains substantial, prompting ongoing efforts to identify novel therapeutic targets. While several new treatment options have modest benefits or limited applicability, sodium–glucose cotransporter 2 (SGLT) 2 inhibitors have exhibited significant benefits proportional to neurohormonal blockade GDMT.
Sodium–glucose cotransporter mechanism of action and initial development
SGLT proteins exist in many tissues throughout the human body in several isoforms and operate in active glucose uptake into cells and as water channels.6,7 Of the several isoforms, SGLT1 and SGLT2 have been the focus of modern medicine due to known genetic diseases arising from spontaneous mutations in Slc5a1 and Slc5a2 genes, respectively.7 SGLT1 is located in the kidneys, intestines, liver, lungs, brain and heart, while SGLT2 predominates the S1 and S2 segments of the apical brush border membrane of the renal proximal convoluted tubules.8
Both SGLT1 and SGLT2 actively facilitate reabsorption of glucose by cotransport of sodium down its electrochemical gradient into the cell. However, the coupling of sodium and glucose for the two channels is variable, 2:1 for SGLT1 and 1:1 for SGLT2.9 With these dynamics, SGLT2 reabsorbs approximately 90% of the filtered glucose in the kidneys, with SGLT1 in the S3 segment reabsorbing the remainder.10 The absolute percentage is variable due to the sequential nature of glucose reabsorption in the kidneys.
Owing to their fundamental role in renal glucose reabsorption, inhibitors for SGLT1 and SGLT2 were developed over the past decade as a novel class of antidiabetic medications. Phlorizin is a botanical compound that was first isolated from fruit trees in 1835 and has since benefitted pharmaceutical research. Its primary mechanism of action is avidly inhibiting SGLT1 and SGLT2, resulting in renal glycosuria while avoiding hypoglycaemia.11 Although employing this characteristic for diabetes management is ideal, phlorizin is not fitting as clinical pharmacotherapy because it is poorly absorbed from the gastrointestinal tract.11 However, phlorizin’s potential led to the development of SGLT inhibitors, with a particular focus on SGLT2 inhibitors, which serve as the main site for glucose reabsorption.
As SGLT2 inhibitors were developed and studied as antiglycaemic therapy, researchers uncovered certain characteristics from this class of medications. The pharmacodynamics of SGLT2 inhibitors are not affected by other diabetes medications.12 The onset of action is approximately 1–2 hours, and the offset of action, based on dose, is roughly 24–48 hours.13 Additionally, the fractional excretion of glucose achieved by SGLT2 inhibition remains constant, despite patient size and degree of insulin resistance. With these features, the average glucose reabsorption prevented by SGLT2 inhibitors is in the range of 30–50%, driven by the intrinsic potency of the particular SGLT2 inhibitor.6
The expanding role of SGLT2 inhibitors from diabetes treatment to HF management is fortuitous. In 2007, Nissen and Wolski published a meta-analysis demonstrating a significantly increased risk of myocardial infarction associated with the use of rosiglitazone.14 In response to this study, the US Food and Drug Administration (FDA) has required assessment of cardiovascular outcomes and safety for new diabetes medications.15 Since then, randomized controlled trials with diabetes medications have been designed as non-inferiority studies compared to placebo, with a primary outcome composite of major adverse cardiovascular events (MACE) combining cardiovascular mortality, non-fatal myocardial infarction and non-fatal strokes.16
Initial sodium–glucose cotransporter 2 inhibitor trials for diabetes management indicating cardiovascular benefit
In the past decade, investigators have tested a number of different SGLT inhibitors in large-scale randomized clinical trials to evaluate their efficacy in diabetes management and to observe the cardiovascular safety profile. The SGLT2 inhibitors, or gliflozins, in clinical use possess varying affinities for the SGLT2 cotransporter. Empagliflozin is highly selective for SGLT2 with SGLT2:SGLT1 binding of approximately 2,700:1; whereas canagliflozin, with the lowest selectivity, has only a 160-fold higher affinity for SGLT2. Dapagliflozin, one of the more frequently used SGLT2 inhibitors for HF, has a >1,200-fold affinity for SGLT2 over SGLT1.17 Conversely, sotagliflozin is essentially a non-selective SGLT inhibitor, binding SGLT2:SGLT1 with a 20:1 ratio.18 Discussed below are the major initial trials examining cardiovascular safety with SGLT2 inhibitors, which are also summarized in Table 1.19–27
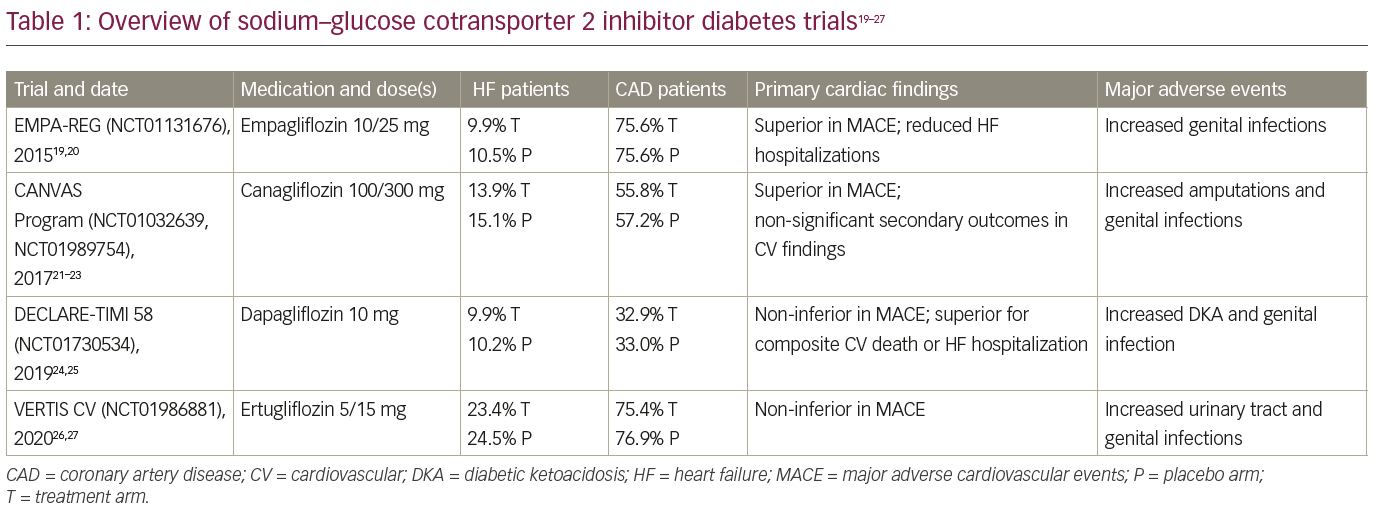
In late 2015, Zinman et al., in their EMPA-REG OUTCOME trial (NCT01131676), published the first randomized controlled trial of empagliflozin and its cardiovascular safety after FDA approval.19,20 This trial compared the effects of cardiovascular morbidity and mortality in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease on standard-of-care therapy; approximately 10% of patients were diagnosed with HF at enrolment. A total of 7,028 patients in 42 countries were randomly assigned to receive empagliflozin 10 mg or 25 mg daily or placebo in a 1:1:1 fashion. For the first 12 weeks, other antiglycaemic therapy could not be adjusted, but after that period, investigators were encouraged to augment therapy if needed to improve glycaemic control. The primary outcome was a MACE composite, as required by the FDA, and the secondary outcome was a composite of the primary outcome and hospitalization for unstable angina. The primary outcome occurred in a significantly lower percentage of patients on empagliflozin (10.5%) compared with placebo (12.1%) over a median follow-up of 3.1 years, meeting criteria for superiority and driven by a reduction in cardiovascular death. The secondary outcome met criteria for non-inferiority, occurring in 12.8% treated with empagliflozin compared to 14.3% on placebo. Other key findings included reduced all-cause mortality (5.7 versus 8.3%) and HF hospitalizations (2.7 versus 4.1%) with empagliflozin. Adverse events were similar between the groups except for more genital infections occurring in the empagliflozin groups.
The CANVAS Program published its results in 2017, examining the safety of canagliflozin in patients with diabetes with established, or high risk for, atherosclerotic cardiovascular disease.21 At enrolment, 14.4% of patients carried a diagnosis of HF. This study combined data from two related trials. An initial trial (CANVAS; NCT01032629) was introduced in December 2009, prior to FDA approval for the use of the medication.22 After canagliflozin became the first SGLT2 inhibitor granted FDA approval in March 2013, a second trial (CANVAS-Renal [CANVAS-R; NCT01989754]) was designed in a similar fashion to meet the revised post-approval FDA requirement.23 The data from the two trials were pooled to examine a primary outcome of MACE and secondary outcomes of death from any cause, cardiovascular death, albuminuria, and a composite of cardiovascular death and HF hospitalizations. A total of 10,142 patients were involved (4,330 in CANVAS and 5,812 in CANVAS-R), 9,734 of which completed the trial. After a 2-week run-in period, participants were randomized to a 1:1:1 ratio in CANVAS, to receive either canagliflozin 100 mg or 300 mg daily, or placebo. The participants in CANVAS-R were randomized 1:1 to canagliflozin 100 mg daily or placebo, with an option to increase canagliflozin to 300 mg daily after 13 weeks (of which, 71.4% participants in the canagliflozin group did). Canagliflozin demonstrated superiority for the primary outcome: 26.9 events per 1,000 patient-years compared with 31.5 events for placebo. The secondary outcomes did not meet statistical significance; however, there was a signal towards reduction of cardiovascular death and HF hospitalizations (16.3 versus 20.8 events per 1,000 patient-years) and albuminuria (89.4 versus 128.7 events per 1,000 patient-years) with canagliflozin treatment. A new finding of increased amputations, especially at the toe or metatarsal level, was noticed with canagliflozin use, but other adverse effect rates were as expected.21
Dapagliflozin was the first SGLT2 inhibitor approved in the world for clinical use by the European Union in 2012. Its cardiovascular safety outcomes in patients with diabetes with established, or at high risk for, atherosclerotic cardiovascular disease were reported in 2019 by the DECLARE-TIMI 58 investigators (NCT01730534);24,25 approximately 10% of patients were diagnosed with HF at enrolment. As per prior trials, the primary safety outcome was MACE. There were also primary efficacy outcomes of MACE and a composite of cardiovascular death or HF hospitalizations, building on the positive results of the EMPA-REG OUTCOME trial.19,20 Secondary efficacy outcomes included a renal composite, as well as death from any cause. A total of 25,698 patients enrolled initially, of which, 17,160 completed a 4- to 8-week run-in period with placebo alone and then were randomly assigned 1:1 to dapagliflozin 10 mg daily or placebo. Dapagliflozin was non-inferior to placebo for the primary safety outcome of MACE, 8.8% for dapagliflozin compared with 9.4% for placebo over a median of 4.2 years. For the primary efficacy outcomes, dapagliflozin was not superior to placebo in terms of MACE, but did meet criteria for superiority for the composite of cardiovascular death or HF hospitalization (4.9% versus 5.8% in placebo). Of note, this composite was fundamentally driven by a reduction in HF hospitalization, as there was no difference in cardiovascular death between the two groups. Furthermore, there were significantly fewer renal events in the dapagliflozin group. Overall safety and adverse events trended towards higher rates of diabetic ketoacidosis and genital infections in the dapagliflozin group, compared with the placebo.24
The VERTIS CV trial (NCT01986881), published in 2020, describes cardiovascular outcomes for the more-recently approved ertugliflozin.26,27 A total of 8,238 patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease were randomized into groups receiving ertugliflozin 5 mg or 15 mg daily or placebo on a 1:1:1 ratio; 23.7% of patients had a history of HF. The ertugliflozin groups were combined for outcomes analysis. Ertugliflozin was non-inferior to placebo in terms of the primary outcome of MACE; 11.9% for both, over a median follow-up of 3.5 years. The secondary outcome of cardiovascular death or HF hospitalization was not significantly different between the groups. Adverse events were similar to other SGLT2 inhibitors, with higher urinary and genital infections and numerically higher, but not significantly higher, rates of amputations and diabetic ketoacidosis.26
These initial studies were designed, based on the rosiglitazone experience, to detect increased risk of myocardial infarction and other atherosclerotic events, and enrolled patients at risk for such events; HF was not initially considered a priority. Furthermore, patients with chronic HF only comprised a small portion of the study populations and were not further characterized by functional class or ejection fraction. Interestingly, the earlier trials (EMPA-REG and CANVAS) displayed superiority in MACE, but the subsequent trials did not. Differences among the trial populations likely contributed to this variation; the prevalence of coronary disease ranged from 33% in DECLARE-TIME 58, to 76% in both EMPA-REG and VERTIS CV. A variety of other factors may also have contributed, including trial design variances, differences in SGLT2 inhibitors, more intensive secondary therapy for MACE, and others. It is interesting that the benefits noted were independent of the glycated haemoglobin (HbA1c)-lowering effects of the medications. Outside of these, a number of other SGLT2 inhibitors have been developed and are being tested globally, such as ipragliflozin (NCT02479399), luseogliflozin (NCT02500186), tofogliflozin (NCT02201004) and remogliflozin (NCT02537470).28–31
Presumed cardiovascular mechanism of action and benefit of sodium–glucose cotransporter 2 inhibition
SGLT inhibition has been postulated to be beneficial in cardiovascular disease through a variety of mechanisms. The obvious benefit of improved diabetes control aside, the glycosuria also results in weight loss from caloric wasting, and waist circumference reduction, leading to a reduction in insulin resistance; however, the weight loss from SGLT2 inhibitors is modest, averaging 1.5–2 kg.32 This may also be related to suggestive reductions in epicardial adipose tissue, and hence, coronary atherosclerosis; though one would expect to realize the benefits of this mechanism over years.33 Moreover, there are known overwhelming changes in myocardial insulin signalling, secondary to generalized insulin resistance, resulting in myocardial dysfunction. This is surmised to be due to reduced insulin-mediated uptake and utilization of glucose for required contractility of the myocardial cells. It is believed that chronic pathologic conditions, such as HF, result in euglycaemic hyperinsulinaemia, prompting resistance in those without diabetes.34 Modulation of insulin resistance, and hence, myocardial insulin signalling, may be a potential benefit of SGLT2 inhibitors as well.34
By increasing urinary glucose and sodium excretion, there is accompanying osmotic diuresis, which results in decreased preload and augments the effects of other diuretics. One small study demonstrated a significant reduction in pulmonary capillary wedge pressure, but no improvement in cardiac index after 12 weeks of empagliflozin use.35 There are some suggestions, that with prolonged SGLT2 inhibition, energy dynamics undergo changes similar to those seen with aestivation. This physiologic state required to survive arid hot conditions promotes water-conservation systems and switches to fatty acid utilization and ketogenesis to counteract fuel loss in the form of glycosuria, ultimately enhancing myocardial energetics.36 There is evidence of improved erythropoiesis with SGLT2 inhibitor use, resulting in better end-organ oxygenation.37
Hyperuricemia is associated with increased risk of cardiovascular disease; SGLT2 inhibitors promote augmented uric acid excretion via increased glucose excretion and inhibit its reabsorption via the GLUT9 isoform 2 transporters.38 Along with glycosuria, sodium excretion is an expected effect of SGLT inhibition, resulting in reduced systolic blood pressure. Hypertension has long been recognized as a major risk factor for the development of HF, though, as with the epicardial adipose tissue discussion above, one would expect to realize this benefit after years of therapy. In addition, SGLT2 inhibitors have also been shown to reduce inflammation and high-sensitivity C-reactive protein (CRP) levels.39 Both the reduction in systolic blood pressure and high-sensitivity CRP are associated with reduced vascular resistance and arterial stiffness.39 All three of these parameters are known to result in improved cardiovascular outcomes. Further posited mechanisms that may be beneficial include desirable changes in myocardial metabolism and reduction in cardiac fibrosis.40 In summary, further study is needed to understand which of these mechanisms is responsible for the beneficial clinical effects of SGLT2 inhibitors.
Sodium–glucose cotransporter 2 inhibitor trials for heart failure management
After the initial randomized controlled trials revealed not only superior outcomes in MACE but also suggested reduced HF hospitalizations, several trials have been conducted to explore the benefits of SGLT2 inhibitors, specifically in patients with HF (Table 241–46 and Figure 1). The DAPA-HF trial (NCT03036124) was published by McMurray et al. in November 2019.41,42 The HF inclusion criteria were an ejection fraction of ≤40%, New York Heart Association (NYHA) functional class II–IV symptoms and a range of N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels based on last HF hospitalization and atrial arrhythmias.
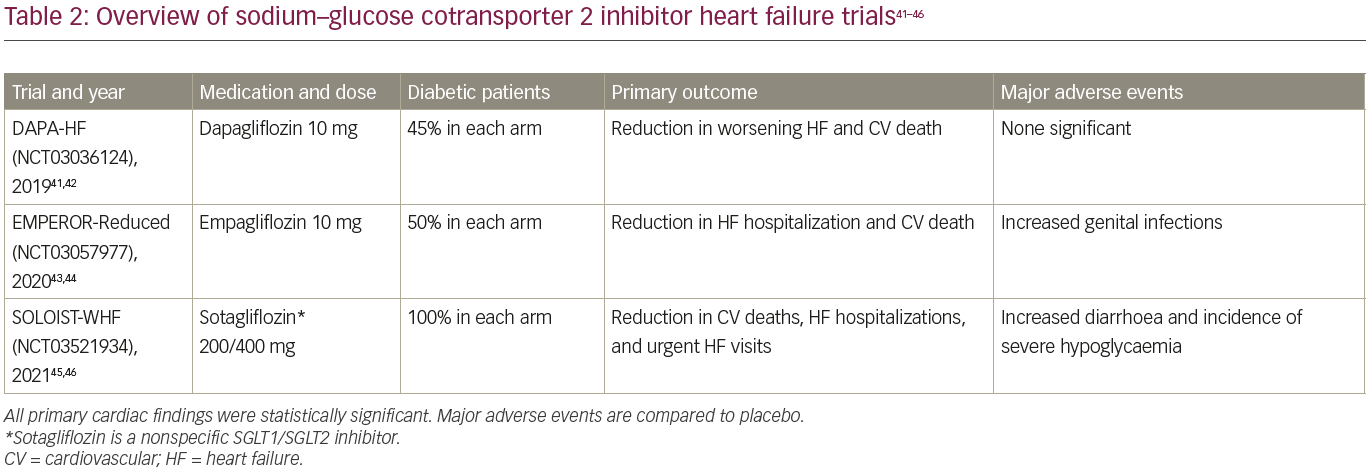
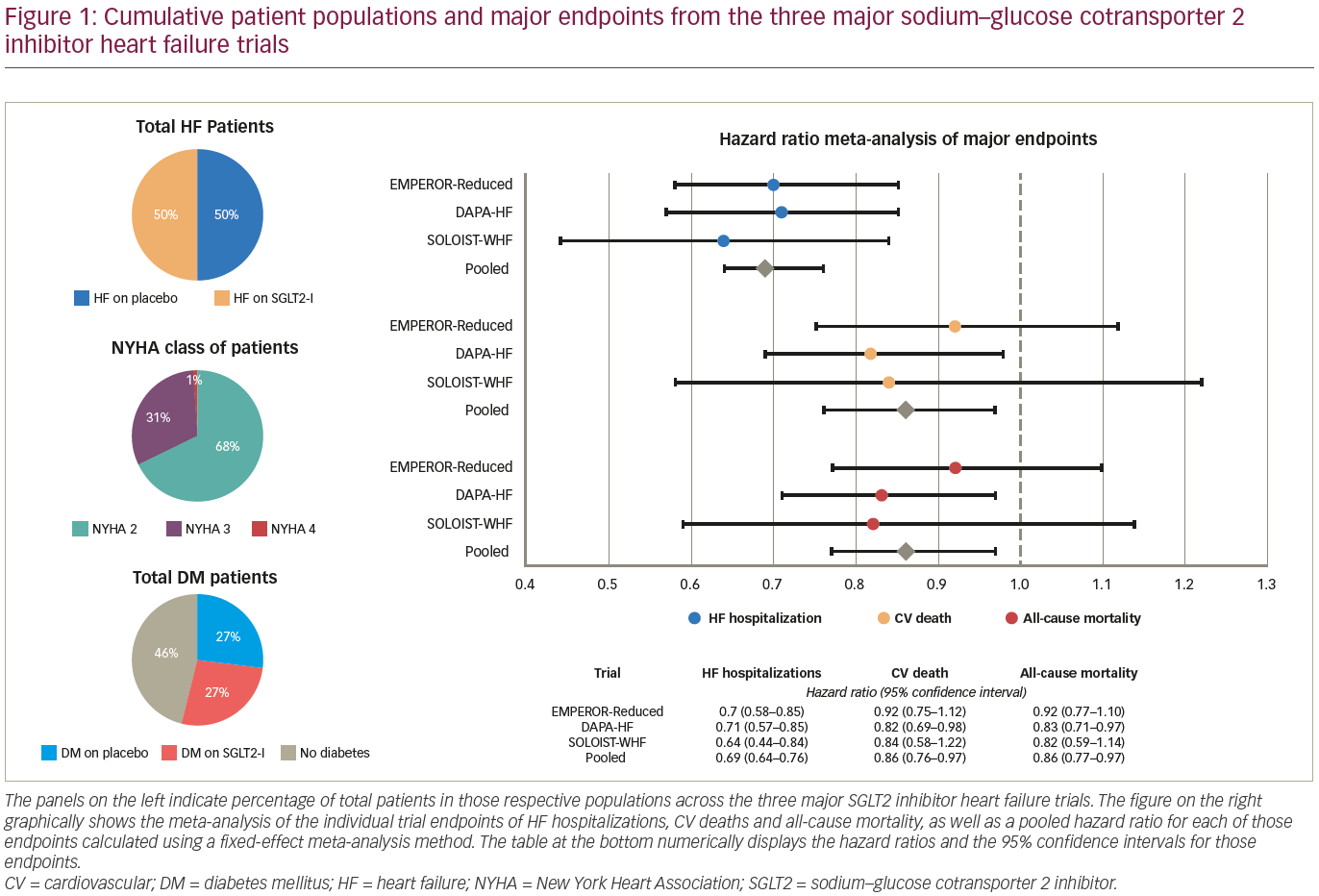
The use of ACE-I/ARB/ARNI and beta blockers was required unless contraindicated, MRA was encouraged, and implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy (CRT) devices were required per guidelines. A diagnosis of diabetes mellitus was not required for enrolment; 41.8% of patients had diabetes. A total of 4,744 patients across 20 countries were randomly assigned to dapagliflozin 10 mg daily or placebo. These patients were well-managed with 96% prescribed beta blockers, 84% ACE-I/ARB, 10.7% ARNI and 71% MRA. Device utilization was less common: 26% had ICDs and 7% CRT devices, presumably due to variations in local guidelines for device implantation.
The primary outcome, a composite of worsening HF (hospitalization or urgent visit requiring intravenous therapy) or cardiovascular death, occurred in 16.3% of patients in the dapagliflozin group and 21.2% of patients in the placebo group over a median follow-up of 18.2 months, significant with p<0.001. Moreover, all three components significantly favoured the use of dapagliflozin, including in patients without diabetes. The secondary outcome, a composite of HF hospitalization or cardiovascular death, and Kansas City Cardiomyopathy Questionnaire (KCCQ) assessing HF symptoms, also favoured dapagliflozin use. Serious adverse events, such as volume depletion and renal events, were not significantly different between placebo and intervention arms; diabetic ketoacidosis and genital infections were more common in the dapagliflozin arm.41 Another dapagliflozin study, DEFINE-HF, a small-scale, short-term, randomized controlled trial performed by Nassif and colleagues, also showed significant improvement in KCCQ scores and HF symptoms, though the co-primary endpoint of improved 6-minute walk distance was not met.47
The EMPEROR-Reduced investigators performed a similar trial assessing the utility of empagliflozin for patients with HF with or without diabetes (NCT03057977).43,44 The HF inclusion criteria were similar to DAPA-HF, though the NT-proBNP levels were different. Nearly half of the patients had diabetes. Patients were assigned in a 1:1 ratio to receive either empagliflozin 10 mg daily or placebo. The primary outcome was a composite of cardiovascular death or HF hospitalization. The key secondary outcomes were the occurrence of all adjudicated hospitalizations (first and recurrent) for HF and rate of decline in estimated glomerular filtration rate. A total of 3,730 patients were enrolled. Similar to DAPA-HF, beta blocker use was 95%, ACE-I/ARB 70%, ARNI 19% and MRA 71%, while cardiac devices were more common (31% had ICDs and 12% CRT devices). The primary outcome occurred in 19.4% of empagliflozin versus 24.7% of placebo patients over a median follow-up of 16 months (p<0.001), and was driven by reduction in HF hospitalizations. As previously seen, genital infections were more common with empagliflozin. Of particular interest, adverse events and safety concerns commonly appreciated with GDMT for HF, such as hypotension, renal dysfunction and electrolyte abnormalities, were not more common with empagliflozin use.43 In the EMPA-TROPISM trial (NCT03485222), 84 patients with HF with reduced ejection fraction (HFrEF) without diabetes were treated with empagliflozin 10 mg daily for 6 months.48,49 Investigators found significantly reduced left ventricular (LV) end-systolic and diastolic volumes by cardiac magnetic resonance imaging, the primary endpoint, as well as increased LV ejection fraction (LVEF), reduced LV mass and spherical remodelling, increased peak oxygen consumption, and enhanced quality of life by KCCQ scores.48,49 A similar positive impact on reverse LV remodelling with empagliflozin use was detected in patients with HF and diabetes in the SUGAR-DM-HF trial (NCT03485092).50,51
The SOLOIST-WHF trial (NCT03521934), published in January 2021, examined the usefulness of the non-selective SGLT inhibitor, sotagliflozin, in patients with diabetes during a HF hospitalization with elevated BNP or NT-proBNP and requiring IV diuretic therapy. Importantly, there were no LVEF criteria.45,46 Patients enrolled were clinically stable, did not require supplemental oxygen or intravenous therapies and had systolic blood pressure 100 mmHg or higher. A total of 1,222 patients (79.1% with ejection fraction <50%) were randomized to receive sotagliflozin 200 mg daily (increased to 400 mg daily depending on adverse reactions) or placebo before (48.8% of participants) or within 3 days (51.2%) of hospital discharge. GDMT was excellent; 92% prescribed beta blockers, 64% MRA, 83% ACE-I/ARB and 17% ARNI. Because trial enrolment closed early after loss of funding, the primary outcome was changed to a composite of the total number of cardiovascular deaths, HF hospitalizations and urgent HF visits. Primary outcome analysis discovered sotagliflozin use to be favourable (51.0 versus 76.3 events per 100 patient-years, p<0.001), and was consistent across multiple pre-specified subgroups, including LVEF ≥50%. The patients on sotagliflozin, compared with those on placebo, did suffer from increased diarrhoea (6.1% versus 3.4%) and severe hypoglycaemia (1.5% versus 0.3%). While this trial was limited due to loss of funding, the trial did suggest benefit of SGLT inhibition in managing recently decompensated HF and HF with preserved EF (HFpEF).
The future of sodium–glucose cotransporter 2 inhibition and concluding remarks
While the benefit of SGLT2 inhibition for diabetes management has long been theorized, improved cardiovascular outcomes were not expected. Several major studies involving thousands of patients have clearly shown the utility of SGLT2 inhibitors in improving cardiovascular outcomes, especially HF. Benefits have been seen with multiple SGLT2 inhibitors, suggesting a class effect. Ongoing and future studies (EMPERORPreserved [NCT03057951] for empagliflozin and DELIVER [NCT03619213] for dapagliflozin) are examining the potential benefit of SGLT2 inhibitors in HFpEF, building on the SOLOIST-WHF trial.44–46,52 Moreover, the recently published studies such as the CREDENCE (NCT02065791), DAPA-CKD (NCT03036150) and SCORED (NCT03315143) trials demonstrate benefits of SGLT inhibition for chronic kidney disease, but they are beyond the scope of this review.53–58
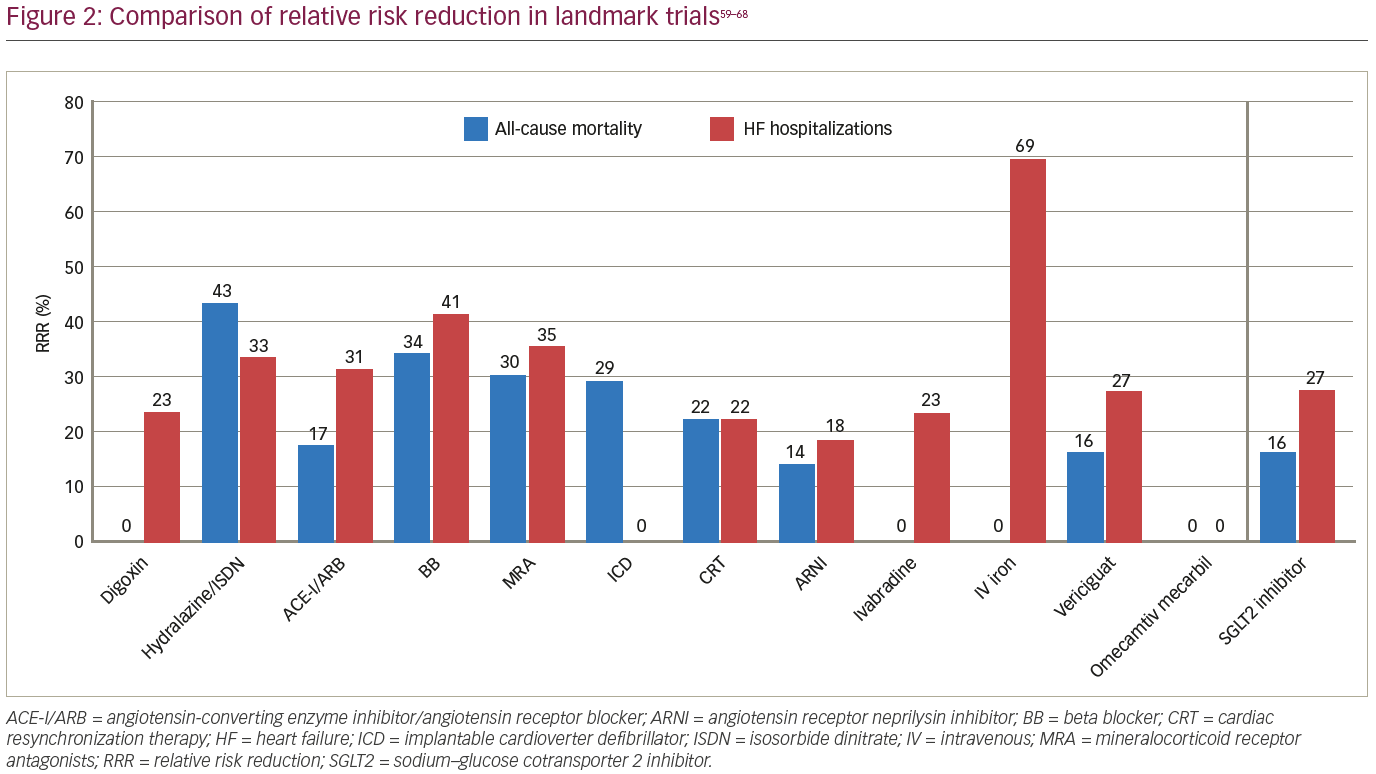
Given the dramatic evidence obtained thus far supporting the benefit of SGLT inhibitors in HFrEF, this class of medications must be incorporated into a new backbone of GDMT. The magnitude of benefit of HF interventions can be understood by comparing the relative risk reduction (RRR) of all-cause mortality and HF hospitalizations in landmark clinical trials (Figure 2).59–68 In terms of mortality benefit, hydralazine and isosorbide dinitrate showed a 43% RRR in treatment-naïve patients, beta blockers 34%, ACE-I/ARB 17% and MRA 30%.59 More recent medical and device therapies expect patients with HF to be on GDMT with at least beta blockers and ACE-I/ARB for their trials. With this in mind, ARNIs have displayed an impressive additional 14% RRR in mortality, ICDs 29%, and CRT 22%.60–62 With such significant cumulative mortality benefit, contemporary medications have only displayed RRR in HF hospitalizations, such as a 23% RRR with ivabradine and 8% with vericiguat.64,65 Despite all of the benefit derived from these therapies, SGLT2 inhibitors, using the landmark DAPA-HF trial, present a dramatic further mortality RRR of 16% and HF hospitalization RRR of 27%.41
With an extremely favourable risk–benefit ratio, SGLT2 inhibitors should be among the first medications initiated for patients. In the recently published 2021 update to the 2017 American College of Cardiology Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment, the authors suggest adding SGLT2 inhibitors in conjunction with beta blockers, ACE-I/ARB/ARNI (preferred) and MRA in all patients with HFrEF (ejection fraction <40%) except those with contraindications: type 1 diabetes, dialysis, lactation and known hypersensitivity to the medication.69 While many of the adverse effects of using SGLT2 inhibitors are mild and quickly diagnosed, the rare adverse effect of euglycaemic diabetic ketoacidosis does exist and can be fatal due to difficulty in early diagnosis and treatment. Insulinopenia with SGLT2 inhibition of the proximal tubules results in altered kidney ATP utilization and increased glucagon release causing loss of bicarbonate, increased ketogenesis and near-normal glucose levels.70 However, with increasing awareness to recognize and mitigate this, the benefits of SGLT2 inhibitor use continue to significantly outweigh the risks. Additionally, SGLT2 inhibitors do not have similar issues, such as hypotension and hyperkalaemia, and are renally protective in chronic kidney disease, arguing that this class of medication is an option for those in whom other GDMT use may be restricted. Going forward, there may also be enough evidence to recommend SGLT2 inhibitors for the management of HFpEF, but that remains to be seen.