Atrial fibrillation (AF) is one of the most common cardiac diseases in elderly patients and is associated with increased morbidity and mortality. It is of great importance to treat patients with AF with oral anticoagulants (OACs) to prevent ischaemic strokes, which represents the most relevant complication of AF. For many decades, vitamin K antagonists (VKAs) have been the only available OAC. By increasing use of OACs in patients with AF from the early 90s until the early 2000s, rates of ischaemic stroke have decreased significantly, without a clear rise of haemorrhagic strokes.1 Nevertheless, the concerns for cerebral bleeding complications are one major reason for the omission of anticoagulation in patients with AF.
During the last decade, four novel non-vitamin-K oral anticoagulants (NOACs), sometimes referred to as direct oral anticoagulants (DOACs), have been introduced and investigated in patients with AF: dabigatran, rivaroxaban, apixaban and edoxaban. Convincing randomised controlled trials (RCTs) comparing NOACs to VKAs have shown at least a similar efficacy with a comparable to reduced bleeding risk.2–5 Patient adherence was expected to be higher in a drug that does not have to be dose adjusted by regular international normalised ratio (INR) controls and that is not dependent on vitamin K intake. These results have facilitated the hope for reducing ischaemic strokes by increasing the proportion of patients with a regular intake of anticoagulants.
As NOACs have now been available for a couple of years, data from real-world settings and comparisons to the initial RCTs are available. The aim of this review is to address whether these real-world data support our expectations of NOACs.
Randomised controlled trials
RCTs have been conducted to compare NOACs to the gold-standard medication of VKAs; bleeding risk and ischaemic events have been compared for stroke prevention in patients with AF being at risk of stroke represented by the CHADS2 or CHA2DS2-VASc score. Connolly et al. performed an RCT comparing dabigatran (150 mg twice daily [BID] and 110 mg BID) to the VKA warfarin (the RE-LY-trial).2 ROCKET-AF, an RCT by Patel et al., compared rivaroxaban (20 mg once daily [OD]) to warfarin,4 and in the ARISTOTLE trial by Granger et al., apixaban (5 mg BID) was compared to warfarin.3 The fourth NOAC, edoxaban, was compared in two dosages (60 mg and 30 mg) to warfarin in the ENGAGE-TIMI 48 trial by Giugliano et al.5
Overview of registries on novel oral anticoagulant use in atrial fibrillation
RCTs represent the highest quality of medical evidence for testing a specific treatment or medication while minimising the risk of a bias by confounders or systematic differences between treatment groups. However, the collected data might not be representative for a real-world setting where multiple concomitant diseases, medications and questionable compliance can all impact the effectiveness of therapy. In the above-mentioned trials, a patient population is present, which might be distinct from the type of patients encountered in daily clinical routine. A patient with AF might be healthy, but another patient may be elderly with chronic illnesses, multimorbidity and indication for multiple medications. Therefore, real-world observational studies can either endorse the results of the RCT and strengthen their validity remarkably, or question them, cumulating in the need for a repetition of the RCT. The results of real-world data studies are therefore much anticipated.
Graham et al. published real-world data of 67,207 Medicare patients on dabigatran with an intake of either 150 mg or 75 mg BID that were compared to the same number of patients on warfarin.6 Patients >65 years old with AF and without any other reason for OAC (such as pulmonary embolism or thrombosis), were included. Propensity score matching was used to ensure comparable groups. Interestingly, 16% of patients received a reduced dosage of 75 mg BID, that is not approved in Europe for this indication. Moreover, the trial lacked laboratory data on creatinine clearance, so it remains uncertain whether patients treated with this lower dosage actually had severe renal insufficiency.
Yao et al. performed a retrospective analysis of privately insured and Medicare Advantage patients in the USA and compared 14,307 patients on dabigatran, 7,695 patients on apixaban and 16,175 on rivaroxaban each to the same number of patients on warfarin.7 Adult patients with non-valvular AF and OAC were included into this analysis. Of note, 10% of patients in the apixaban group and 16% in the dabigatran group had a CHA2DS2-VASc score of 0 and 1, respectively, and therefore no clear indication for OACs. The follow-up of all patients was relatively short with a mean duration of 0.5–0.7 years.
Coleman et al. published the results of the REVISIT-US study in 2016.8 This was a retrospective study using a USA Market scan database to compare 11,411 rivaroxaban users and 4,083 apixaban users each to the same number of warfarin users by a 1:1 propensity score matching. Patients ≥18 years with non-valvular AF and a CHA2DS2-VASc score ≥2, were included. In this cohort, 17.5% of rivaroxaban and 15.5% of apixaban patients received a reduced dosage (15 mg rivaroxaban OD or 2.5 mg apixaban BID).
In a meta-analysis by Ntaios et al., all high-quality, retrospective, real-world studies were summarised, including 136,221 patients on rivaroxaban, 606,855 patients of dabigatran and 66,482 patients on apixaban.9 As endpoint definitions differed between the published studies, a direct comparison of all included patients was difficult.
With respect to edoxaban, published real-world data were sparse until now. The results of the ongoing observational international study ETNA-AF,10 regarding real-world data on edoxaban, are much awaited. It is hoped that this study will confirm findings of a nationwide Korean observational study by Yu et al., that investigated a limited number of 2,840 patients on high-dose edoxaban (60 mg) and 3,016 patients on low-dose edoxaban (30 mg) and compared them each to a propensity score matched VKA group.11
Overview of similarities and differences of randomised controlled trials and real-world data with respect to endpoints
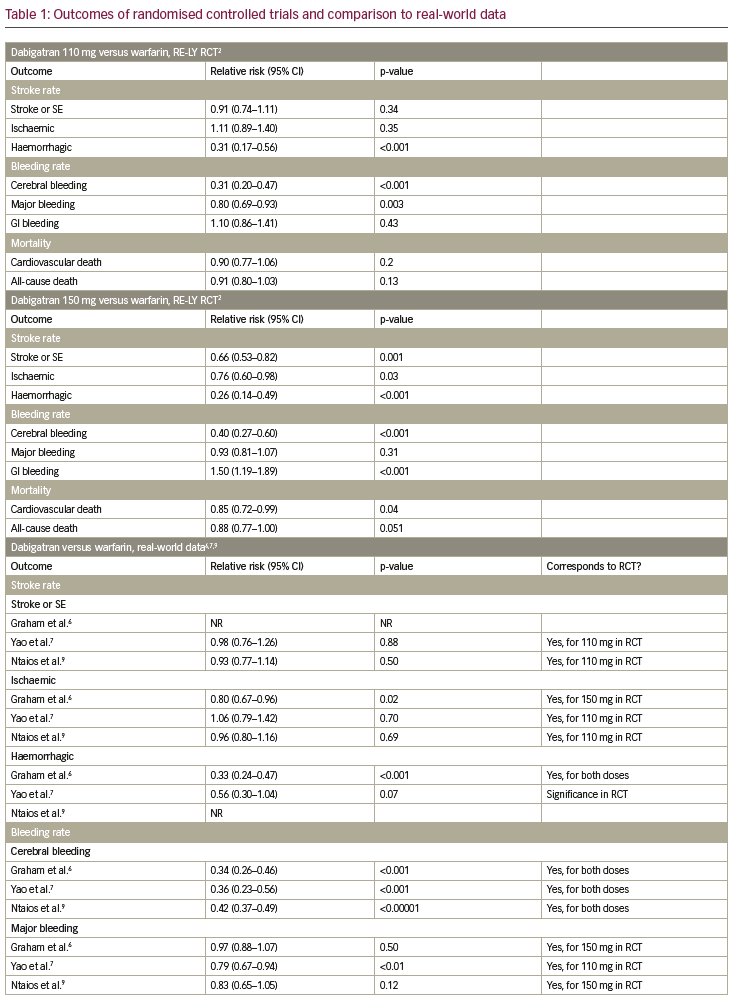
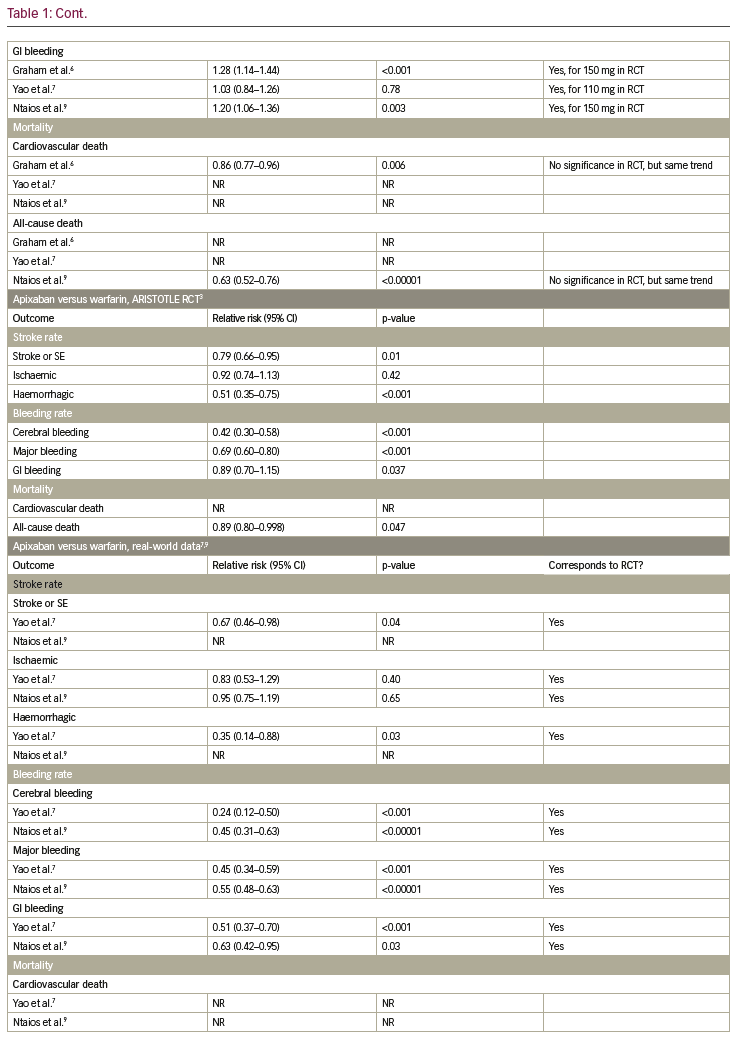
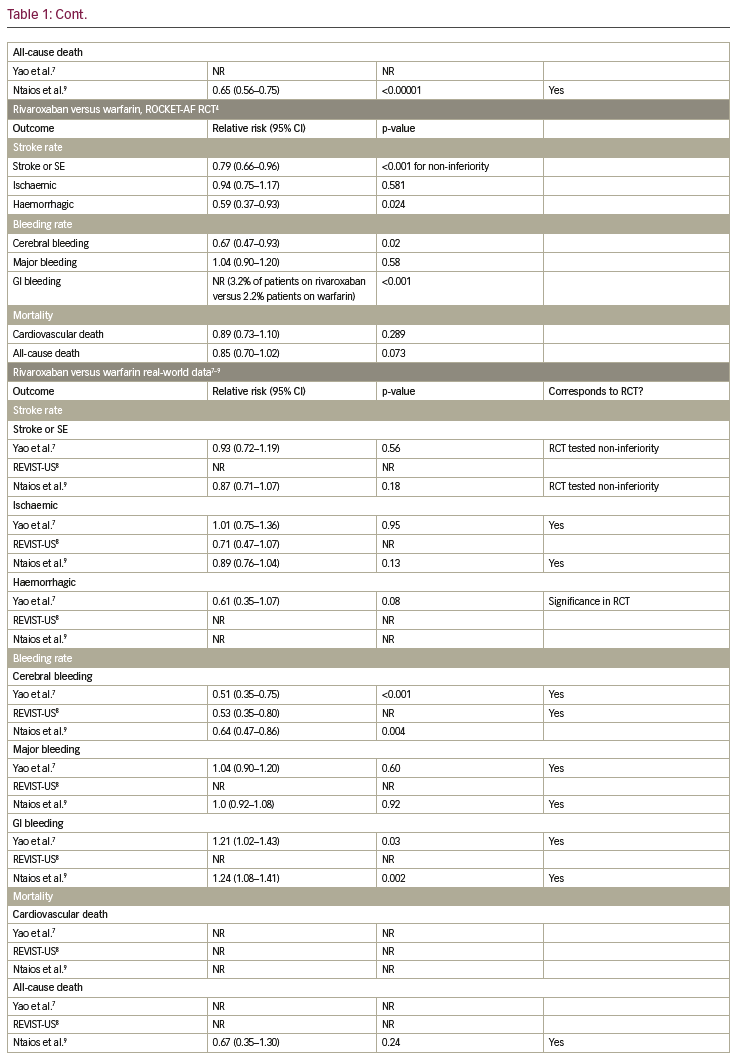
Table 1 summarises the results of the RCTs and compares them to available real-world data. In the following paragraphs, each endpoint is described separately.
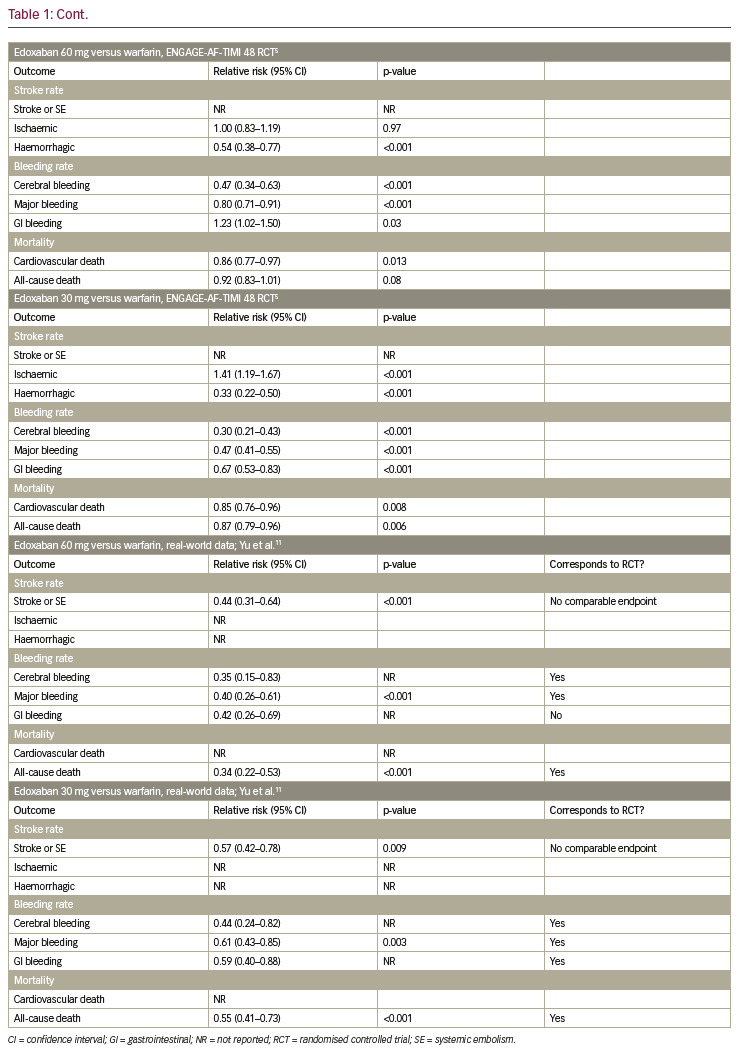
Stroke and systemic embolism
In the RE-LY trial, dabigatran 150 mg was shown to reduce the rate of stroke and systemic embolism significantly, while dabigatran 110 mg did not show a significant reduction, when compared to warfarin.2 In the observational trial by Yao et al. and the meta-analysis by Ntaios et. al., dabigatran did not lower the incidence of stroke and systemic embolism significantly.7,9 For apixaban, a significant reduction of stroke and systemic embolism was shown, when compared to warfarin in the ARISTOTLE trial.3 This finding was confirmed in the observational trial by Yao et al. and the meta-analysis by Ntaios et al.7,9 In the ROCKET-AF trial, non-inferiority for rivaroxaban compared to warfarin was shown concerning stroke or systemic embolism.4 In the observational trial by Yao et al. and the meta-analysis by Ntaios et al., no significant reduction of stroke and systemic embolism could be shown.7,9 This specific endpoint was not tested in the ENGAGE-AF-TIMI trial,5 but in the nationwide Korean study by Yu et al., a significant reduction of stroke and systemic embolism events was observed in edoxaban-treated patients compared to VKA.11
Ischaemic stroke
In the RE-LY trial a significant reduction of ischaemic stroke was observed for dabigatran 150 mg; whereas there was no significant reduction in ischaemic stroke for dabigatran 110 mg when compared to warfarin.2 In the Medicare data analysis by Graham et al., a significant reduction of stroke was shown in the dabigatran group, while in the observational trial by Yao et al. and the meta-analysis by Ntaios et al., no significant difference could be observed.6,7,9 For apixaban and rivaroxaban, neither in the ARISTOTLE trial (or the ROCKET-AF trial, respectively), nor in the observational trial by Yao et al. and the metaanalysis by Ntaios et al. reported a significant reduction of ischaemic stroke.3,4,7,9 In the ENGAGE-AF-TIMI trial, ischaemic strokes occurred significantly more often in patients treated with edoxaban 30 mg when compared to warfarin.5 For the 60 mg dosage group of edoxaban there was no difference.
Haemorrhagic stroke
The incidence of haemorrhagic strokes was significantly reduced in dabigatran (both dosages) when compared to warfarin in the RE-LY trial.2 This was confirmed in the observational trial by Graham et. al, but not by Yao et al., where no significant reduction could be observed.6,7 In the ARISTOTLE trial, as well as in the observational trial by Yao et al., haemorrhagic strokes occurred significantly less often in patients treated with apixaban when compared to warfarin.2,7 Additionally, for rivaroxaban, a significant reduction of haemorrhagic strokes could be shown in the ROCKET-AF trial, which was not confirmed by the observational trial by Yao et al.4,7 The ENGAGE-AF-TIMI trial showed a significant reduction of haemorrhagic strokes in patients on edoxaban, independent of dosage.5
Cerebral bleeding
Cerebral bleeding rates were significantly lower in patients on dabigatran (both dosages) when compared to warfarin in the RE-LY trial.2 This was confirmed in the real-world observational data by Graham et al. and Yao et al., as well as in the meta-analysis by Ntaios et al.6,7,9 Likewise, for apixaban, rivaroxaban and edoxaban cerebral bleeding rates were reduced significantly in the ARISTOTLE, ROCKET-AF and ENGAGE-AF-TIMI trials when compared to warfarin,3–5 which was confirmed in the observational study by Yao et al. and the meta-analysis by Ntaios et al., for apixaban and rivaroxaban, and the nationwide Korean study by Yu et al. for edoxaban.7,9,11
Major bleeding
Concerning major bleeding, a significant reduction could be shown for dabigatran 110 mg but not for dabigatran 150 mg when compared to warfarin (RE-LY trial).2 A trend towards fewer events in the dabigatran group was also shown in the observational study by Yao et al., but not in the Medicare data by Graham et al. or in the meta-analysis by Ntaios et al.6,7,9 A significant reduction of major bleeding was observed in patients on apixaban, when compared to warfarin, in the ARISTOTLE trial,3 which was confirmed in the observational trial by Yao et al. and the meta-analysis by Ntaios et al.7,9 For rivaroxaban, no significant reduction could be shown whether in the ROCKET-AF-trial, nor in the observational study by Yao et al. or the meta-analysis by Ntaios et al.3,4,7,9 In the ENGAGE-AF-TIMI trial, a significant reduction of major bleeding was shown, which was confirmed in the nationwide Korean observational trial.5,11
Gastrointestinal bleeding
Rates of gastrointestinal (GI) bleeding were significantly elevated in patients on dabigatran 150 mg, but not in the 110 mg dosage group, when compared to warfarin in the RE-LY trial.2 This significant increase in GI bleeding rates could also be seen in the observational study by Graham et al. and the meta-analysis by Ntaios et al.6,9 However, the observational study by Yao et al. did not show a significant difference in GI bleeding rates for dabigatran in comparison to VKA.7 In the ARISTOTLE trial, a significant reduction in GI bleeding was shown for apixaban,3 which was in line with the results of the observational study by Yao et al. and the meta-analysis by Ntaios et al.7,9 For rivaroxaban, significantly higher GI bleeding rates were observed in the ROCKET-AF trial and confirmed in the observational trial by Yao et al. and the meta-analysis by Ntaios et al.3,4,7,9 In the ENGAGE-AF-TIMI trial, significantly higher GI bleeding rates were observed for edoxaban 60 mg, whereas significantly lower GI bleeding rates were shown for edoxaban 30 mg.5 In contrast, in the nationwide Korean observational study, GI bleedings occurred less often in both dosage groups.11
Cardiovascular death
For dabigatran 110 mg, no significant reduction of cardiovascular death was shown, whereas for dabigatran 150 mg, a significant reduction of cardiovascular death was observed in the RE-LY trial.2 In the observational data by Graham et al., a clear and significant reduction of cardiovascular death in patients on dabigatran could be detected.6 This endpoint was not investigated in the ARISTOTLE trial, but a significant reduction of cardiovascular death was shown in the meta-analysis by Ntaios et al.2,3,9 For rivaroxaban, no significant reduction of cardiovascular death was shown in the ROCKET-AF trial, while this endpoint was not addressed for rivaroxaban in the observational studies.4 In the ENGAGE-AF-TIMI trial, a significant reduction of cardiovascular death was observed for both edoxaban dosages.5
All-cause death
For dabigatran, no significant reduction in all-cause death was observed in the RE-LY trial.2 However, in the meta-analysis by Ntaios et al., a significant reduction could be shown.9 For apixaban, in the ARISTOTLE trial, a significant reduction in all-cause death was shown.2,3 In line with this finding, the meta-analysis by Ntaios et al. also showed a significant reduction in all-cause death for patients treated with apixaban versus VKA.9 In the ROCKET-AF trial as well as the meta-analysis by Ntaios et al., no significant reduction of all-cause death was observed for patients treated with rivaroxaban.4,9 In contrast, for edoxaban 30 mg, a significant reduction of all-cause death was shown in the ENGAGE-AF-TIMI trial and confirmed in the nationwide Korean observational trial, in which a significant reduction of this endpoint was observed for both dosages.5,11
Myocardial infarction
For dabigatran, rates of myocardial infarction were investigated in the RE-LY trial.2 They were significantly higher in dabigatran 150 mg in comparison to warfarin. However, in the huge meta-analysis by Ntaios et al. analysing 66,090 patients on dabigatran, the dabigatran patients had a similar rate of myocardial infarctions compared to patients on VKAs without signs of a pro-ischaemic risk.9
Problems with novel oral anticoagulant use in daily life
Severe renal insufficiency
One of the reasons why patients with AF still receive VKA therapy is kidney failure. Apixaban, edoxaban and rivaroxaban have been approved for patients with renal insufficiency and a creatinine clearance of over 15 mg/dl, and dabigatran has been approved for a creatinine clearance of over 30 mg/dl. A retrospective analysis by Siontis et al., investigated patients with severe renal insufficiency and apixaban intake. Only patients on dialysis at the time of OAC prescription were included. The analysis compared 2,351 patients on apixaban to 7,053 patients on warfarin. No statistical difference in event rates of stroke/systemic embolism and all-cause death could be shown; however, patients on apixaban suffered significantly fewer major bleeding events (p<0.001). Of note, patients on high-dose apixaban (5 mg BID) had significantly fewer event rates of stroke/systemic embolism and all cause death, as well as major bleeding, when compared to patients on warfarin.12 Chan et al. retrospectively investigated 281 patients on dabigatran and 244 patients on rivaroxaban with dialysis in 2015. There was no significant difference in hospitalisations or death from bleeding between the two groups. The authors, however, described that one of limitations of this study was that the numbers of included patients and occurred events were too low to detect meaningful differences.13
Concomitant dual antiplatelet therapy
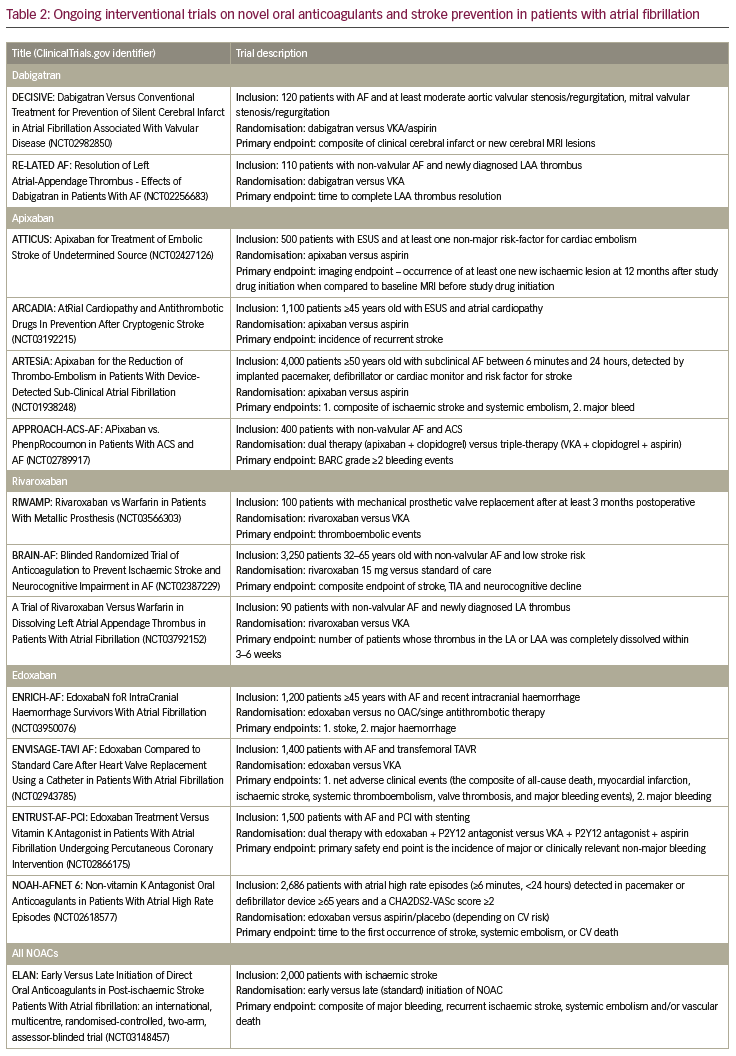
There have been a couple of RCTs in recent years investigating the use of NOACs in a triple-therapy collective (e.g. patients with AF and stenting, who are therefore in need of a combination of OACs and anti-platelet therapy). Mainly, these trials have tested the use of dabigatran,14 rivaroxaban,15 and apixaban16 instead of VKAs in a dual-treatment strategy with a P2Y12 inhibitor against a standard triple therapy with a VKA, a P2Y12 inhibitor and aspirin. The extremely high risk of bleeding of up to 44% annually17 was reduced in all triple-therapy trials with omitting aspirin and only treating patients with a dual-therapy concept with a NOAC (instead of VKA) and a P2Y12 inhibitor.14–16 The ongoing ENTRUST-AF percutaneous coronary intervention trial will answer the question of a dual therapy with edoxaban and a P2Y12 inhibitor in the context of AF and stenting, instead of triple therapy.18 It is of great importance that none of those already released trials were powered for a potentially higher risk of thromboembolic events (e.g. stent thrombosis), when omitting aspirin. Also, results of RCTs investigating patients with a sole acute coronary syndrome collective are missing, just as real-world data, confirming those results (see Table 2 for ongoing trials on NOACs).
Elderly patients
A great proportion of daily-treated patients belong to a high-risk collective, for example because of high age and increased tendency to fall, advanced renal insufficiency and multiple comorbidities and co-medications.19 These patients have always been, and still are, underrepresented in RCTs, as the mean age of patients in RCTs comparing NOACs to VKA is between 70–73 years and the CHADS2 score, indicating the stroke risk, is low to moderate in those trials (from 2.0 in the ARISTOTLE trial, up to 3.5 in the ‘ROCKET-AF’ trial).2–5 This makes it complex to find the best possible treatment option for elderly and high-risk patients. Therefore, it is important to focus on these high-risk patients, that we treat in our daily clinical routine, in future trials.
Mechanical valves
Real-world data of observational and retrospective studies that were released in recent years mostly confirm the findings of the initial RCTs and strengthen them further.2–9,11 Still, VKAs are the hallmark of anticoagulation for selected patients with contraindication for NOACs, as well as for stroke and thrombosis prophylaxis in patients with mechanical heart valves. In this context of mechanical heart valves, a sole clinical trial with NOACs was performed and stopped prematurely due to exaggerated stroke and bleeding risks.20 For this indication, VKAs, therefore, remain the gold standard.
Dosing problem in real-world patients
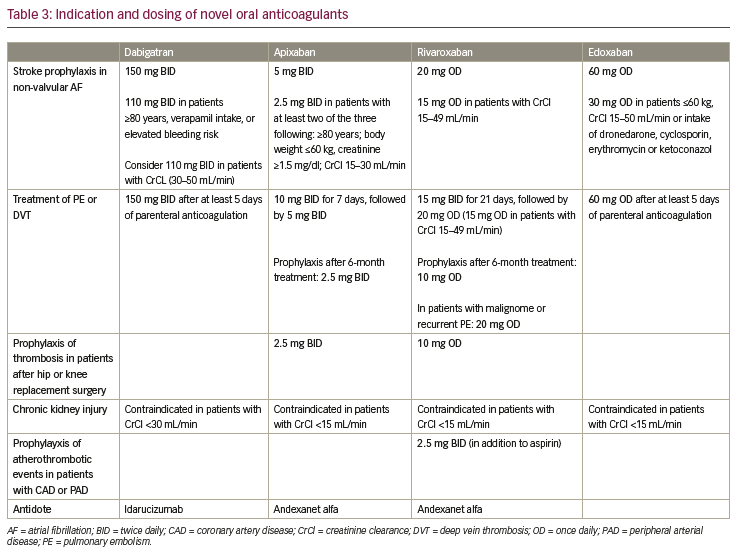
One of the problems that appears in real-world data trials is inadequate dosing. In RCTs, patients are treated with the intended dosage of their study drug (see Table 3 for NOAC dosing for different indications). In a real-world setting, a not-irrelevant number of patients is treated with an inadequately low drug dosage for multiple reasons. This makes it harder to compare real-world data to the initial pivotal trials. In the RE-LY trial, dabigatran was tested in two fixed dosages (150 mg BID and 110 mg BID), in the real-world trial 16% of dabigatran users received a reduced dosage (75 mg BID) despite the fact that only 33% of those patients had the diagnosis of a chronic kidney disease, and only 20% of those, a severe kidney disease (and therefore the indication for dose reduction).6 Data from the ORBIT-AF II trial concerning dose reduction were investigated and presented by Steinberg et al. In this analysis, 6,636 (84%) out of 7,925 patients on NOACs received the full dosage, which was an adequate dosing in 96% of the cases. The remaining 1,289 patients (16%) received a reduced dosage of their NOAC.21 Interestingly, in only 43% of those cases, the dose reduction was consistent with US Food and Drug Administration labelling and recommendations. Patients on rivaroxaban received an appropriately reduced dosage in 52%; whereas, patients on apixaban had an appropriate dose reduction in only 37% of the cases. Thus 63% of apixaban-treated patients received a reduced dosage without indication. Patients in those groups with inappropriate dose reduction for rivaroxaban or apixaban did not show a higher rate of thromboembolic events and all-cause mortality.21 Regarding edoxaban, an analysis of baseline characteristics and dose adherence was already published prior to the end of the awaited ETNA-AF trial.10,22 In 13,474 European patients, dose reduction was performed in 23.3%,22 which is in line with the patients with dose reduction in the ENGAGE-AF-TIMI 48 trial.5 But if all globally investigated patients (24,431) are taken into account, a dose reduction was performed in 43.1%, which is a much higher number than reported in the RCT.22
Patients undergoing atrial fibrillation ablation
Patients undergoing ablation for AF are typically on OAC treatment. The major complications in ablation procedures comprise stroke and transient ischaemic attacks. For the reduction of those, systemic anticoagulation before, during and after ablation is necessary. During the ablation procedure, heparin can be administered; maintaining the activated clotting time >300 seconds, before and after ablation OAC is recommended for at least 8 weeks.23 In the COMPARE trial, uninterrupted VKA therapy was shown to be superior to bridging with heparin concerning stroke and minor bleeding rates.24 For the four NOACs, four RCTs were performed evaluating the risk for bleeding and stroke in uninterrupted NOAC use versus uninterrupted intake of VKA. Cappato et al. showed in the VENTURE AF trial that uninterrupted intake of rivaroxaban and VKA do not result in significantly different bleeding or stroke rates.25 For dabigatran, Calkins et al. reported a significant reduction of major bleedings with continuous dabigatran versus continuous VKA intake in patients undergoing AF ablation in the RE-CIRCUIT trial. The stroke rate was very low and no statistically significant difference between the treatment groups could be observed.26 Kirchhof et al. showed in the AXAFA AFNET 5 trial that continuous apixaban intake compared to VKA intake is safe and effective in patients undergoing AF ablation with respect to stroke and bleeding.27 Consistently, the same was shown for edoxaban; Hohnloser et al. investigated continuous edoxaban intake versus continuous VKA intake in patients undergoing AF ablation in the ELIMINATE AF trial. Similar event rates with regard to bleeding and stroke events could be observed for either treatment strategy.28
Do real-world data support our expectations?
Evidence-based guidelines recommend OAC for patients with AF and risk factors for stroke.29,30 Nevertheless, this effective way of preventing strokes is still underused.31 The main reasons for not prescribing OACs are a history of bleeding or the tendency to fall – both resulting in the fear of major bleedings.32 After the RCTs and real-world data trials, discussed above, NOACs were expected to reduce the number of patients with AF suffering a stroke or a severe adverse reaction like a major bleeding event under OACs. Probably based on these results, the use of NOACs increased worldwide in recent years from 4.2% (from March 2010 to June 2012) to 37% (from June 2014 to June 2015), with a consecutive fall of VKA-use, but a rise of total anticoagulation from 57.4% (from March 2010 to June 2012) to 71.1% (from June 2014 to June 2015), as described in the GARFIELD-AF registry.33 Notably, the number of patients with OAC rose in all levels of risk (stratified by CHA2DS2-VASc score), including those patients with a score of 0, probably indicating overtreatment. Further analysis showed a rise of NOAC prescription, particularly in elderly patients or patients with dementia, which might previously not have received an adequate treatment.33
Conclusion
After the introduction of the four NOACs: dabigatran, rivaroxaban, apixaban and edoxaban, RCTs were performed to ensure their efficacy and safety when compared to the gold-standard VKAs. The results of the RCTs had a huge impact and already significantly changed daily clinical practice. Subsequently, real-world data from different patient groups are available now. These data sets show that the results of RCTs are also largely reflected in a real-world setting. Due to these convincing results, the guidelines on anticoagulation in patients with newly diagnosed AF were changed and a NOAC treatment should now be preferred over a VKA treatment. This underlines the importance and the great value of NOACs in our daily clinical practice.