Atrial fibrillation (AF), one of the most common cardiac arrhythmias, is considered to be a significant risk factor for cardiovascular disease, stroke and mortality. The prevalence of AF increases with age, and patients with AF are at risk of atrial thrombosis and its consequences.1 The pathogenesis of AF consists of inflammatory activity, structural remodelling and endothelial dysfunction.2 It is estimated that more than 10% of patients with AF in the USA are undiagnosed,3 and this shows the importance of finding predictive markers for AF.
Inflammation that occurs in the atrium is due to the infiltration of inflammatory cells and is associated with an increase in inflammatory markers such as C-reactive protein, osteoprotegerin, vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1).4 VCAM-1 and ICAM-1 are endothelial adhesion molecules (EAMs) expressed on the activated endothelial cells, belonging to a family of immunoglobulin-like molecules. These two EAMs have increased expression in inflamed microvessels and are responsible for the adhesion and migration of monocytes and lymphocytes.5
VCAM-1 expression is associated with various cardiac diseases, such as heart failure, and rheumatic and ischaemic heart diseases.6,7 Reactive oxygen species and haemodynamic factors enhance cardiac VCAM-1 expression, which causes sustained cardiac remodelling, fibrosis and dysfunction.8,9 Atrial upregulation and increase in VCAM-1 expression has been reported in patients with AF.10 It has been demonstrated that blockade of angiotensin II receptor reduces the occurrence of AF, and the proposed mechanism is the downregulation of adhesion molecules within the atrium.11 Two studies have reported high levels of serum VCAM-1 in patients with AF compared with sinus rhythm (SR);4,12 however, another study showed no difference in this regard.13 In addition, there are conflicting findings in studies regarding the correlation between the increased expression of VCAM-1 and the promotion of thrombogenic factors and left atrial appendage clot formation.14–16 ICAM-1 expression has been detected in the blood vessels of the atrium and the atrial endocardium.17 On the other hand, similar vascular and muscular expressions of VCAM-1 and ICAM-1 have been seen in patients with postoperative atrial fibrillation (POAF) and patients without arrhythmia, such as those with coronary artery disease.18 Furthermore, higher
ICAM-1 levels have been found to be linked to hypertension.19
To the best of our knowledge, no systematic review and meta-analysis has been conducted to summarize the relationship between VCAM-1/ICAM-1 and AF; therefore, we aimed to assess the effect of VCAM-I and ICAM-1 on AF and whether these EAMs could be used as biomarkers to identify patients at high risk of AF, including after cardiac surgery.
Methods
This study was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).20 The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO).21
Eligibility criteria
The inclusion criteria for this study were: (1) patients with AF as the study population; (2) reports of VCAM-1 and ICAM-1 serum levels. The exclusion criteria were: (1) other types of articles such as case reports, review articles, letters, comments; (2) languages other than English; (3) conference abstracts; (4) studies conducted on animals.
Search
Two independent researchers (M.R. and S.D.) conducted the search. The literature search was performed through PubMed, Scopus, EMBASE and Web of Science for studies published from January 1990 until April 2022. Search terms in PubMed included: (“vascular cell adhesion molecule-1” [Mesh] OR vascular cell adhesion molecule-1 OR [title/abstract], “intercellular adhesion molecule-1” [Mesh] OR intercellular adhesion molecule-1 [title/abstract]) AND (“atrial fibrillation” [Mesh] OR atrial fibrillation [title/abstract]). Grey or unpublished literature was identified manually by searching bibliographies.
Study selection and data extraction
We used Endnote X6 for organizing the studies. After removing duplicate records, studies were selected based on titles and abstracts by two independent researchers (M.T. and H.M.). Studies were then assessed for inclusion based on the full text, and data were extracted using a table in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). Information was collected regarding study design, studied tissue, tissue staining, number of cases and controls, serum levels of VCAM-1 and ICAM-1, measurement methods, and the type of surgery that preceded the occurrence of AF.
Quality appraisal
We evaluated the quality of the included studies based on the Joanna Briggs Institute (JBI) checklists.22 Two authors (L.N. and M.R.) completed the quality assessments. Any disagreement during the process was resolved by consensus-based discussion or another researcher’s comment (S.D.).
Data synthesis and analysis
The meta-analysis was performed following the Cochrane Collaboration recommendations, and the results were reported following the PRISMA statement.20,23 The data were expressed as mean and standard deviation (SD). We used the formula by Hozo et al. to estimate the mean and SD for the data reported as median (min–max).24 For estimating the SD in articles that reported data as median (Q1–Q3), we used the formula by Wan et al., and the median was considered to be equivalent to the mean.25 The meta-analysis was conducted using Open Meta-Analyst® software (Brown University, RI, USA). We used the mean difference (MD) between patients with AF and those with normal SR to conduct the meta-analysis. A subgroup analysis of studies including only patients with persistent AF was also conducted. In addition, the range of EAMs in patients with AF was calculated using 95% confidence intervals (CIs). Heterogeneity was evaluated by I2 statistics. Significant heterogeneity of results was acknowledged when an I2≥50%. A p-value of <0.05 was considered statistically significant.
Results
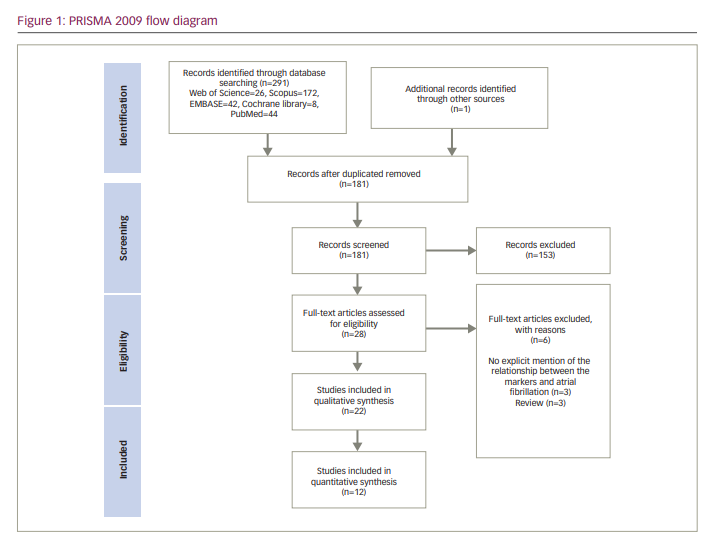
We identified 291 articles through the search from four databases. In addition, one article was found manually through references of other studies. After duplicates were removed, the titles and abstracts of 181 articles were analysed for eligibility. Eventually, 28 articles went through full-text screening, and six articles were excluded because they did not meet our eligibility criteria. The reasons for the exclusion of articles are presented in Figure 1. Finally, we included 22 studies in the systematic review.4,6,10,12–15,17,18,26–38
Tissue expression of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 in patients with atrial fibrillation
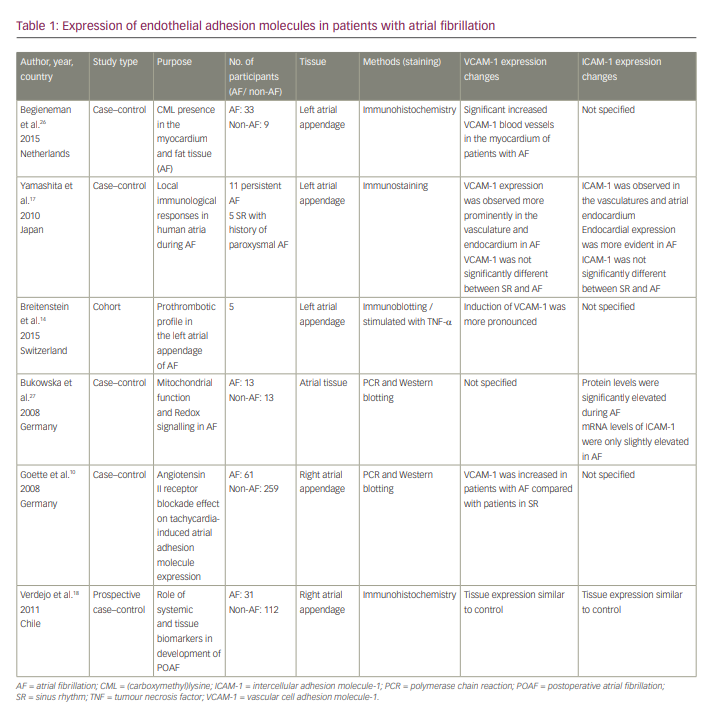
Five articles discussed the changes in expression of VCAM-1 in atrial tissue of patients with AF (Table 1). Three studies14,17,26 found an increase in VCAM-1 tissue expression in the left atrial appendage, while Goette et al.10 found an increase in its tissue expression in the right atrial appendage and Verdejo et al.18 reported similar tissue expression of VCAM-1 in right atrial appendage between patients with AF and the control group. Only three out of six articles reported alterations in ICAM-1 tissue expression in patients with AF. Two studies17,27 reported increased ICAM-1 tissue expression in atrial tissue, and one study18 did not find any increase in ICAM-1 tissue expression in the right atrial appendage.
Serum levels of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 in patients with atrial fibrillation
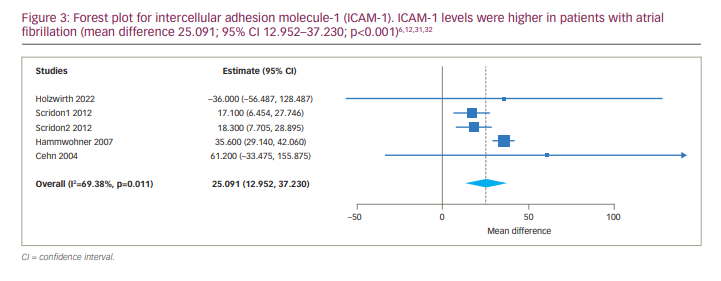
Seven studies assessed serum levels of VCAM-1 and/or ICAM-1 in patients with AF compared with individuals with normal SR (Table 2). Two cohort studies28,29 reported significantly higher levels of VCAM-1 in individuals who developed AF; however, levels of VCAM-1 in these two studies were only reported at baseline and were not specified for AF and SR cases separately. Six studies reported serum levels of VCAM-1 in both groups.4,6,12,13,30,32
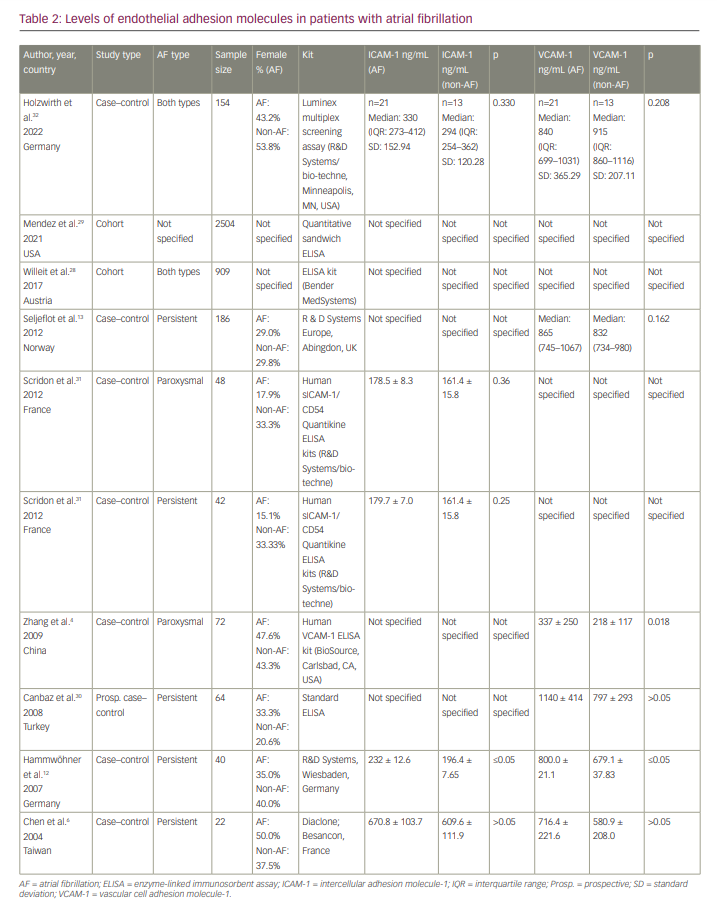
A meta-analysis was carried out, and VCAM-1 levels were higher in patients with AF, with an MD of 86.782 (95% CI 22.805–150.758; p=0.008). The forest plot is depicted in Figure 2. In addition, the analysis revealed significant statistical heterogeneity among studies (p<0.001, I2=85.208); the continuous random-effects model was therefore used. Four studies included persistent AF.6,12,13,30 We performed a subgroup analysis for persistent AF, which revealed a significant difference between serum VCAM-1 levels in AF and SR groups (MD 98.046, 95% CI 26.582–169.510; p=0.007).
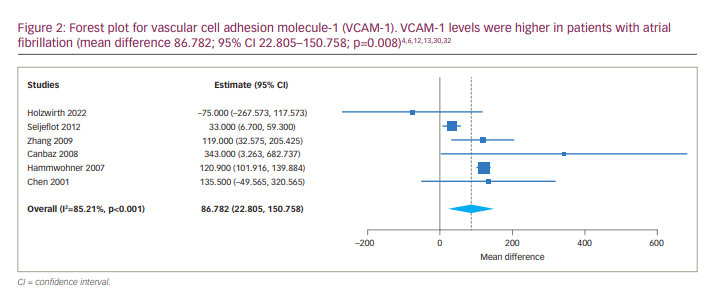
Four studies reported and compared serum levels of ICAM-1 between AF and SR groups. Two of them reported serum levels of ICAM-1 in persistent AF and paroxysmal AF.31,32 Due to the identification of statistical heterogeneity (tau2=96.394, Q=13.063, df (4), p=0.011, I2=69.379%) the continuous random-effects model was used. The results of meta-analysis were as follows: MD 25.091, 95% CI 12.952–37.230; p<0.001 (Figure 3). Subgroup meta-analysis comparing the level of ICAM-1 between persistent AF and SR groups was also associated with a significant difference (MD 28.439, 95% CI 12.540–44.338; p<0.001).
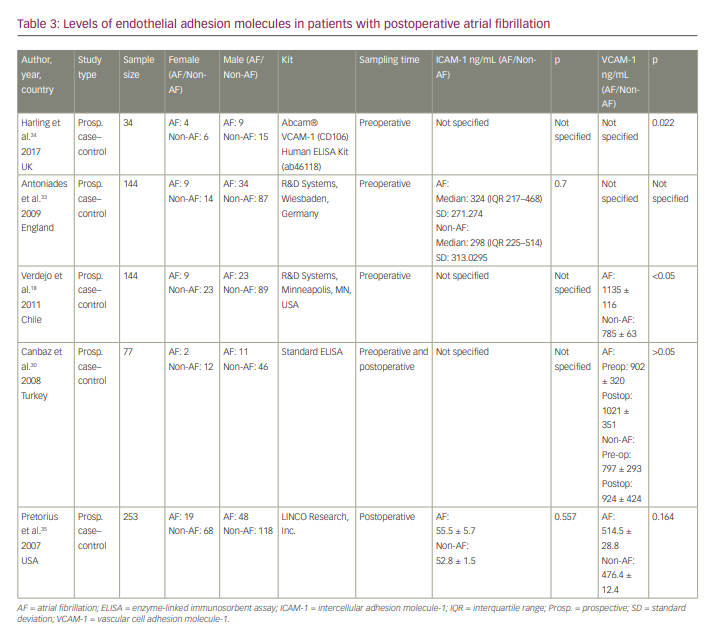
Five articles focused on the relationship of VCAM-1 and/or ICAM-1 with the generation of POAF (Table 3).18,30,33–35 Only two or three reported levels for each of our variables, and because of the great diversity (preoperative and postoperative levels), we decided not to perform a meta-analysis. Only Harling et al. and Verdejo et al. reported a significant difference in serum levels of VCAM-1 between the SR group and the POAF group.18,34 The remaining studies did not report a statistically significant difference between the two groups in terms of serum levels of VCAM-1 and/or ICAM-1.
Range of serum levels of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 in patients with atrial fibrillation
We found 12 articles reporting levels of VCAM-1 and/or ICAM-1 in patients with AF (Table 4). We did not include the studies by Antoniades et al. and Verdejo et al. because their blood sampling for two markers was done preoperatively.18,33 Across nine studies, the 95% CI of VCAM-1 in patients with AF was 661.394 to 927.984 ng/mL (Figure 4). The studies by Ehrlich et al.15 and Holwirth et al.32 did not specify the VCAM-1 levels for persistent and paroxysmal AF patients separately, and they could not be included in the subgroup analysis. The subgroup analysis for persistent AF resulted in a VCAM-1 range of 694.260 to 847.743 ng/mL.
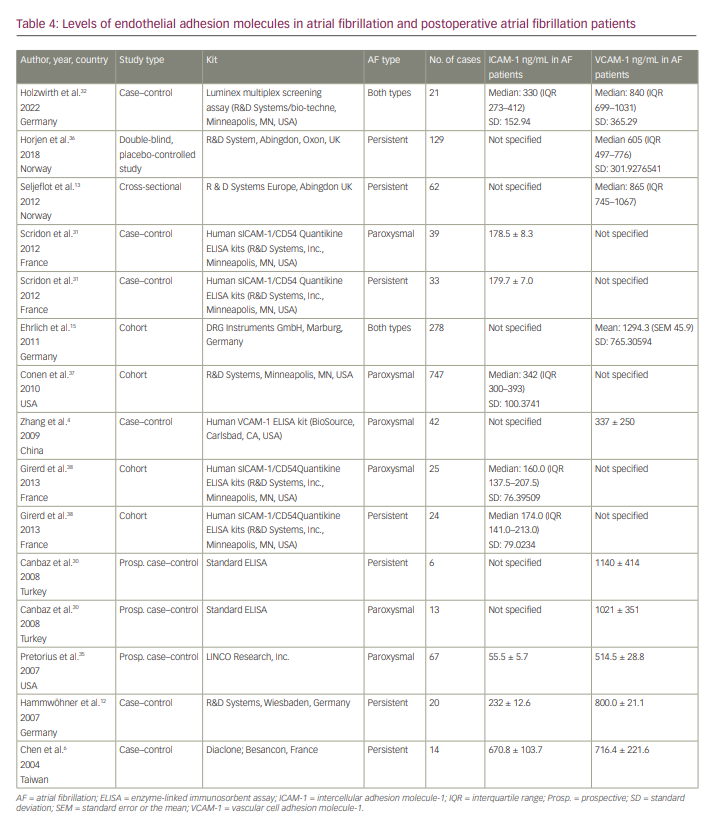
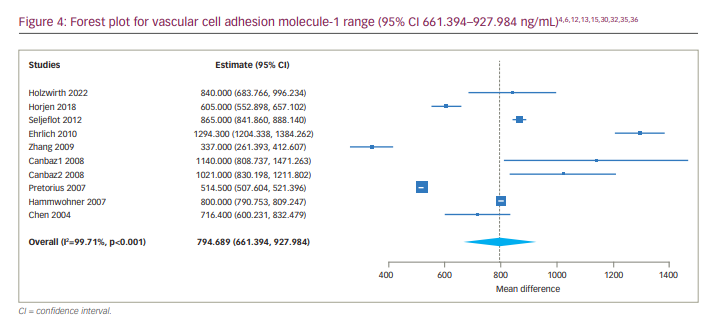
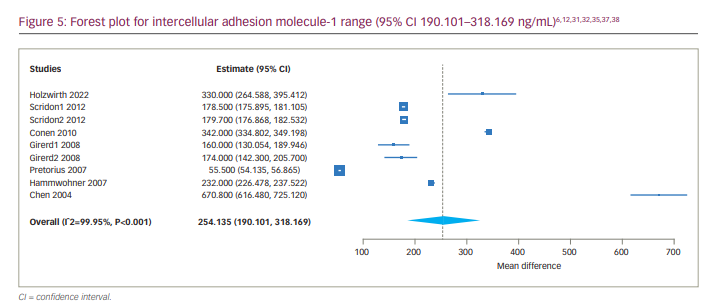
We also determined the 95% CI of the serum levels of ICAM-1 in patients with AF, which was 190.101 to 318.169 ng/mL (Figure 5). We also conducted a subgroup analysis of studies including only patients with persistent AF, which showed a much narrower range of ICAM-1 (95% CI 242.945–349.817 ng/mL).
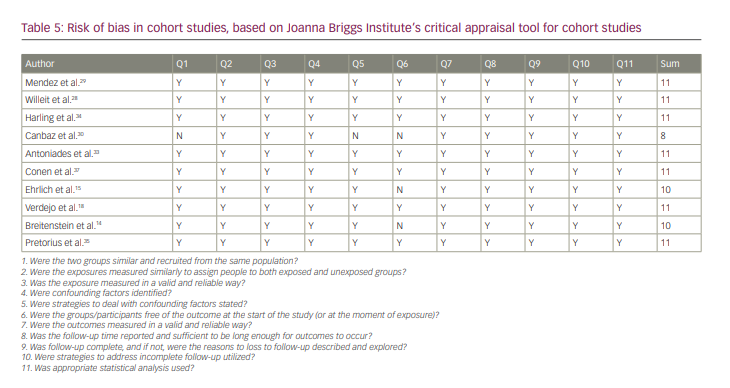
Bias risk within studies
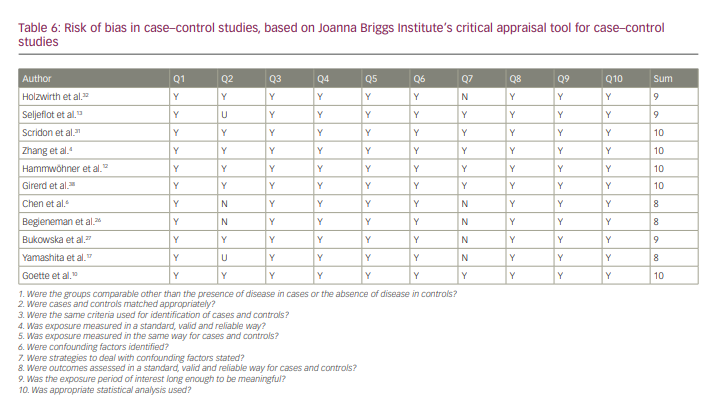
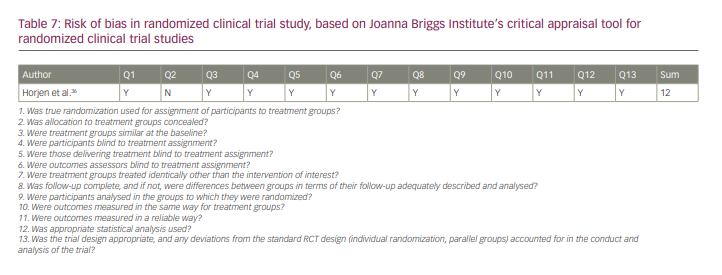
All of the studies were of good quality according to JBI checklists. The results of the quality analysis are presented in Tables 5–7. The mean score for cohort studies was 10.5 (maximum score=11). In addition, 9.18 (maximum score=10) was the mean score for included case–control studies. The study by Horjen et al. was a derivation of the trial, and it was assessed by checklist for randomized controlled trials and scored 12 out of 13.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis evaluating VCAM-1 and ICAM-1 serum levels and tissue expression in patients with AF. In addition, we aimed to examine the effect of these markers in the development of AF in different situations. Our study showed a statistically significant difference between serum levels of the two markers in patients with AF and SR. These results point out that if the cause–effect relationship is proven in future studies, it may be helpful to assess the serum levels of these markers in patients at high risk for AF development, in order to consider the pharmacological and non-pharmacological actions for prevention. We also analysed the serum levels of ICAM-1 and VCAM-1 and reported an estimated range of these markers in patients with AF. In order to do this, we did not include preoperative levels in studies conducted by Antoniades et al.33 and Verdejo et al.18 as these levels were reported when AF diagnosis had not been confirmed.
AF has a high economic burden, which imposes a high cost on patients and the healthcare system. Numerous conditions such as increased age, alcohol consumption, heart failure and low vitamin D levels have been linked to AF. VCAM-1 and ICAM-1 have increased endocardial expression, and this may be the link between the inflammation and prothrombotic states responsible for the development of thrombus in the atrium.39,40
VCAM-1 and ICAM-1 are parts of the immunoglobulin superfamily that are associated with the inflammatory process. These molecules are responsible for cell adhesion and trans-endothelial migration of macrophage-like and dendritic cells.41 In contrast to ICAM-1, studies have suggested non-constitutive expression of VCAM-1 (i.e. it is only induced in the activated endothelium).42 VCAM-1 is upregulated whenever there is inflammation, mediating the adhesion of immune cells to the endothelium.36 Their role in different cardiac diseases is now under research. VCAM-1 is linked to congestive heart failure, coronary artery disease and rheumatic heart disease, and ICAM-1 is associated with hypertension.6,7,19 VCAM-1 has been reported to have an increased expression in patients with AF10,17,26 and is more pronounced when induced by tumour necrosis factor-α (TNF-α).14 Increased levels of inflammatory markers in AF suggest inflammation as one of the major bases of the development and perpetuation of this condition.43 The adhesion of circulating leukocytes to the vascular endothelium leads to leukocyte extravasation during inflammation. This process depends on an interaction between VCAM-1 and ICAM-1 and the leukocytes;40,44 therefore, increased levels of these endothelial factors in inflammatory responses such as AF is not unexpected.
There is controversy regarding the link between EAMs and AF. While Seljeflot et al. reported no association between VCAM-1 and AF in a case–control study consisting of 62 AF cases and 124 SR individuals,13 Zhang et al. found a statistically significant difference between the two groups in serum levels of VCAM-1.4 The study by Holzwirth et al. found lower levels of VCAM-1 but higher levels of ICAM-1 in the AF group compared with the SR group; however, these results were not statistically significant.32 Two studies found a significant difference between AF and SR groups in serum levels of VCAM-1.4,12 Multivariate analysis in the studies by Zhang et al. and Conen et al. found VCAM-1 and ICAM-1, respectively, to be independent factors in AF generation.4,37 One study reported VCAM-1 as an independent predictor for atrial thrombi.12 Ehrlich et al. showed that VCAM-1 is independently associated with myocardial infarction, stroke, peripheral embolism or mortality.15 The high heterogeneity seen in the current meta-analysis may be due to different commercial enzyme-linked immunosorbent assay kits used in included studies.
As shown in Table 6, in some of the included case–control studies, cases and controls did not match appropriately. This was the main point that should be considered when analysing the results of the study conducted by Chen et al.6 This study consisted of three groups. The main group included 19 patients with symptomatic mitral stenosis going through mitral valvuloplasty (four patients with SR and 15 patients with chronic AF), and the authors compared this group with two other groups (22 control patients: 14 healthy individuals with SR and eight patients with chronic lone AF). The first group was a heterogeneous group regarding the serum levels of ICAM-1 and VCAM-1, and they should not have been considered in one group.
Regarding POAF, two studies reported a significant difference in serum levels of VCAM-1 between patients with POAF and the SR group.18,34 Canbaz et al.30 found a difference in VCAM-1 levels in preoperative and postoperative samplings in the SR group, and Harling et al. reported a significant reduction in VCAM-1 levels in both AF and POAF groups 48 hours after surgery. Nevertheless, the interesting point is that Canbaz et al. reported a significant increase, whereas Harling et al. found a reduction in VCAM-1 levels after surgery.30,34 It has been shown that VCAM-1 levels significantly correlate with age and white blood cell count.12,18 These data suggest a complicated mechanism in cardiac surgery that involves inflammation cascades and is closely related to the most important risk factor in the development of AF: age.45 Harling et al. and Verdejo et al. found VCAM-1 to be an independent factor in the generation of POAF.18,34
To our knowledge, a range for VCAM-1 and ICAM-1 has not previously been reported in patients with AF. We analysed all of the studies that reported serum VCAM-1 and ICAM-1 levels in patients with AF (without considering the type of AF) and reported the 95% CIs for serum levels of the markers. The highest levels of VCAM-1 and ICAM-1 were reported by Canbaz et al.30 and Chen et al.,6 respectively, whereas the lowest levels of VCAM-1 and ICAM-1 were reported by Zhang et al.4 and Pretorius et al.,35 respectively.
There are some limitations to our study. First, observational studies cannot prove a causative relationship, and all included studies were observational. Second, the possibility of publication bias was not evaluated because of the small number of studies. Third, only studies in the English language were included; therefore, some valuable sources of evidence may have been missed. Fourth, we could not perform a meta-analysis to assess the relationship of VCAM-1 and ICAM-1 with POAF. Finally, differences in the kits utilized for VCAM-1 and ICAM-1 level assessment were not considered in our final analyses. Several homogeneous studies that have a similar sampling pattern are needed to assess this relationship. Despite these limitations, we were able to conduct this study, and we believe this meta-analysis draws attention to the importance of serum VCAM-1 and ICAM-1 levels in predicting and preventing AF, especially POAF.
Conclusion
This systematic review and meta-analysis suggested a positive relationship between serum VCAM-1 and ICAM-1 levels and AF, especially persistent AF. Furthermore, the expression of these markers is increased in human cardiac tissue. The relationship between these markers and POAF needs to be evaluated with large-scale studies that use the
same methodology.