Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and a major cause of morbidity and mortality; however, an optimal strategy for its management remains somewhat unclear, especially for patients with persistent AF.1
Electrical cardioversion (ECV) is widely used in patients with persistent AF when a rhythm control strategy is pursued. In this real-world, observational study, we aimed to define the success rate of ECV in restoring sinus rhythm (SR) and to identify predictors for sustained SR at 12 and 24 months.
Methods
Ethics
This study was approved by the Health Research Authority and Care Research Wales (England and Wales, UK) (Approval number: 272491). All patients provided informed consent to undergo the procedure described in this study. No further consent was required. The study is reported in accordance with the Strengthening the reporting of observational studies in epidemiology (STROBE) guidelines.2
Patients
Data from patients undergoing ECV at Darent Valley Hospital (Dartford, Kent, UK) were prospectively collected in an ECV database. For the current study, all patients who underwent ECV for the treatment of persistent AF between January 2014 and August 2019 were included in the analysis. Patients had to have experienced persistent AF for a minimum of 2 months and must have been on anticoagulation for at least 4 weeks. Data from patients who reverted to SR before ECV, or patients on warfarin with subtherapeutic international normalised ratios were excluded from the analysis. The outcomes of patients who either declined catheter ablation or were not considered for catheter ablation during the study period were analysed.
Electrical cardioversion
In brief, patients were started on an oral anticoagulant therapy, which was continued for at least 4 weeks before ECV. For patients taking the vitamin K antagonist (warfarin), a therapeutic/stable international normalised ratio was required for 6 weeks prior to ECV.
ECV was performed with the patient under deep sedation with intravenous midazolam and/or propofol administered by a cardiac nurse. Blood pressure and pulse oximetry were continuously monitored during the procedure by an anaesthetist.
A biphasic defibrillator with antero-posterior electrode positioning was used to deliver escalating shock energies as follows: 150 J, followed by two further shocks of 200 J if the first attempt was unsuccessful. A 12-lead electrocardiogram was performed before and after ECV.
Outcomes
The primary endpoints were acute success in restoring SR after ECV and SR maintenance at 12 and 24 months post ECV. The secondary outcomes were predictors of SR such as left ventricular (LV) systolic function, use of antiarrhythmic drugs and ECV complications such as stroke, anaesthetic complications, ventricular fibrillation, bradyarrhythmias, myocardial necrosis, myocardial dysfunction, transient hypotension, post-ECV pulmonary oedema and skin burns.
Statistical analysis
Baseline characteristics were summarized numerically for categorical variables and as median values with 25th and 75th percentiles for continuous variables, according to the success of ECV. Cox proportional hazards models were used to identify factors associated with maintenance of SR following ECV at 12 months and 24 months and are presented as odds ratios (ORs) and 95% confidence intervals (CIs). The data analysis was performed using RStudio version 4.0.0 (RStudio, Boston, MA, USA).
Results
We identified 1,028 patients with persistent AF during the abovementioned study period. A total of 319 patients were excluded because they either spontaneously reverted to SR prior to ECV or declined ECV. A further eight patients were excluded because they underwent catheter ablation during the study period. Therefore, 701 patients were included in the final analysis. The mean age of patients was 71 ± 10.8 years, and 70.2% of them were men. Baseline characteristics of included patients are shown in Table 1.
Immediate and sustained success of electrical cardioversion
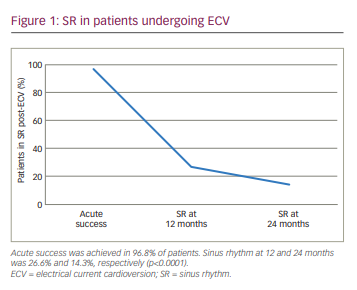
Acute success in restoring SR was achieved in 96.8% of patients. SR at 12 and 24 months was seen in 26.6% and 14.3% of patients, respectively (p<0.0001) (Figure 1).
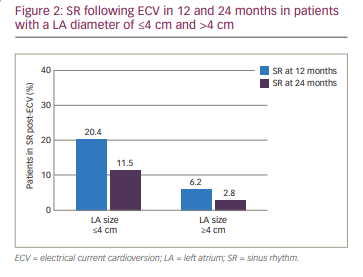
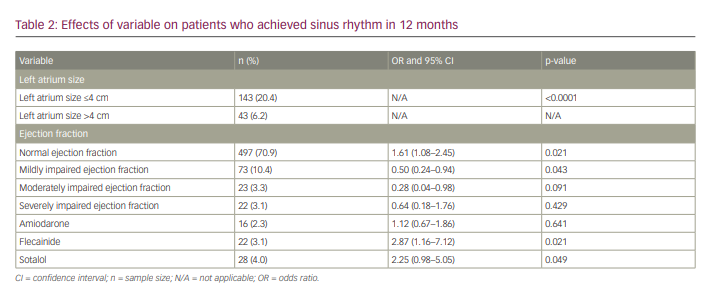
SR was maintained in 20.4% and 11.5% of patients with a left atrium (LA) diameter of ≤4 cm at 12 and 24 months, respectively. Of the patients with an LA diameter of >4 cm, 6.2% and 2.8% maintained SR at 12 and 24 months, respectively (p<0.0001) (Figure 2).
Predictors for sinus rhythm at 12 months
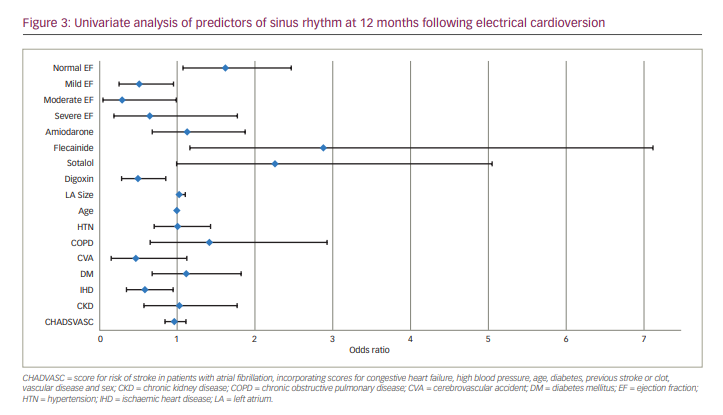
Predictors for sustained SR at 12 months in the univariate analysis were normal LV systolic function and mild LV systolic impairment (OR 1.61, 95% CI 1.08–2.45, p=0.021 and OR 0.5, 95% CI 0.24–0.94, p=0.043, respectively). Flecainide and sotalol therapy improved the chances of SR at 12 months (OR 2.87, 95% CI 1.16–7.12, p=0.021 and OR 2.25, 95% CI 0.98–5.05, p=0.049, respectively). Multivariate analysis revealed no further positive predictors for SR maintenance in 24 months (Table 2, Figure 3).
Adverse events
One patient developed a clinical stroke a few hours following ECV.
Discussion
The restoration of SR using ECV in patients with symptomatic persistent AF can improve cardiovascular haemodynamics, functional status and quality of life.3 In this single-centre retrospective registry study, we investigated the acute and long-term success of ECV in restoring and maintaining SR. The study is unique, as the population comprised patients who did not undergo catheter ablation as part of their treatment, either because they declined the procedure when offered or because they were unsuitable for this treatment; therefore, none of the patients underwent catheter ablation during the study period. Acute success was high at 96.8% and is, therefore, comparable to previous studies, which have reported success rates of between 90.0% and 100.0%.4
Structural heart disease and electrical cardioversion success
Unsurprisingly, patients with normal LA size and/or preserved LV systolic function had better outcomes at both 12 and 24 months. Normal LA size (<4.5 cm) has previously been shown to be a predictor of improved outcomes, which is consistent with our findings.5 In contrast, no correlation between LV systolic function and ECV outcomes has been reported previously, which is inconsistent with our findings.6,7 Of the various factors such as age, sex, arrhythmia duration, hypertension, rheumatic and non-rheumatic mitral valve disease previously investigated as predictors of success and better outcomes post-ECV, only short AF duration has been shown to predict better outcomes.8–10 It is plausible that LA remodelling that occurs with long-duration AF plays a role in LA size, which contributes to poor outcomes post-ECV.
Adjunctive anti-arrhythmic pharmacotherapy
Pre-treatment with flecainide and sotalol increased the chances of staying in SR at 12 months. Flecainide and sotalol can be used in patients without significant structural heart disease, and this may explain why patients who were pre-treated with these agents had relatively good outcomes. Previous studies have reported a modest benefit of amiodarone that is comparable to sotalol in maintaining SR following ECV in persistent AF.11 However, in contrast to these studies and in agreement with a study by Le Son et al., we found that amiodarone did not improve the chances of staying in SR,12–15 perhaps because patients with moderate–severe LV systolic dysfunction or significant structural heart disease are more likely to be given amiodarone rather than flecainide or sotalol for safety reasons.
Complications of electrical cardioversion
ECV is safe and acutely efficacious with few complications. Nevertheless, we recorded one stroke within a few hours following ECV. Other previously reported potential complications include anaesthetic complications, ventricular fibrillation due to lack of synchronization between the direct current shock and the QRS complex, bradyarrhythmias, myocardial necrosis, myocardial dysfunction, transient hypotension, post-ECV pulmonary oedema and skin burns.16
Current role for electrical cardioversion
Catheter ablation is widely available, safe and effective.17 In contrast, ECV has poor long-term efficacy;18 indeed, in our study, only 26.6% and 14.3% of patients remained in SR at 12 and 24 months, respectively. This brings into focus the role of ECV for persistent AF in the current era of catheter ablation availability.
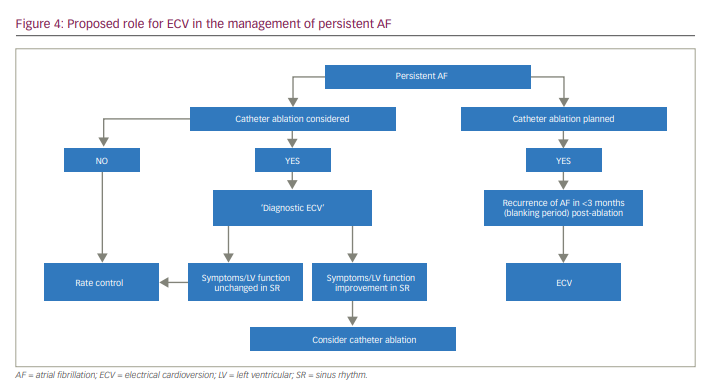
ECV may have a role in determining whether or not patients are truly asymptomatic, sometimes referred to as ‘diagnostic ECV’, selecting patients who can then be referred for catheter ablation. Diagnostic ECV may also be useful to show an improvement of symptoms (or not) when in stable SR and to assess LV function improvement in SR. This information can then be used in determining which patients can be referred for catheter ablation.
ECV is frequently required for early AF recurrence after catheter ablation. One study suggested that early post-ablation ECV failure is not associated with long-term rhythm outcome,19 whereas another found that failed ECV during the blanking period was an independent predictor of future recurrence of AF.20 Outside these specific situations, the role of ECV in long-term maintenance of SR remains poorly defined. We propose a strategy for identifying patients with persistent AF who may benefit from ECV therapy (Figure 4).
Study limitations
This was an observational study that used consecutive patients at a single institution, and the results should be interpreted with caution. The retrospective analysis of databases may have underestimated a number of minor complications following ECV and includes inherent selection bias. We determined the AF duration based on the first electrocardiogram documentation; therefore, patients with asymptomatic AF and/or paroxysmal episodes may have been misclassified.
Conclusions
ECV for persistent AF is acutely efficacious, with a low incidence of major complications. However, during long-term follow-up, recurrence of AF is common, and ECV is not an effective long-term strategy for the maintenance of SR. We propose a strategy for using ECV in the management of persistent AF. Catheter ablation is effective, widely available, and should be the preferred strategy for the majority of patients with persistent AF.