Atrial fibrillation (AF) is the most common type of treated cardiac arrhythmia. The prevalence of the condition increases with age, and it presents with a wide spectrum of symptoms and severity. As the population ages, particularly in developed countries, the burden of AF increases.1 Compared with sinus rhythm, AF is also associated with an increased incidence of mortality, stroke and coronary events, which lead to additional financial burden.2 Thus, AF represents an evolving, global epidemic, and an effective AF therapy is pivotal in reducing morbidity and improving quality of life. Since the publication of a landmark study by Haïssaguerre et al.,3 which provided evidence that AF is frequently triggered by ectopic beats arising from within the pulmonary veins and paved the way towards ablation therapy, AF catheter ablation has become a well-established treatment for the prevention of AF recurrences. Circumferential antral pulmonary vein isolation (PVI) is now paramount to the vast majority of AF ablation procedures.4
A successful PVI requires the creation of a durable, contiguous, transmural and electrically isolating ablation scar lesion encircling the pulmonary veins, causing irreversible tissue injury to the target cardiac tissue while avoiding collateral tissue destruction or injury to the surrounding anatomical structures, such as the oesophagus, the coronary arteries and the phrenic nerve.4,5 Radiofrequency (RF) remains the most common energy source for point-by-point ablation for circumferential PVI, despite the fact that alternative methods and energy sources, such as cryoenergy,6 laser energy,7 microwave energy8 and, more recently, irreversible electroporation,9 are also available. However, despite huge advancements in catheter technology and the development of contact-force (CF)-sensing catheters, pulmonary vein (PV) reconnection, acutely or at more than 3 months after PVI, remains an issue. The overall 1-year success rate for paroxysmal AF ablation ranges between 66%10 and 87%11 in different studies. PV reconnection is the main cause of recurrence of AF or other atrial arrhythmias, and is commonly due to inability to attain transmural or contiguous lesions, either acutely or in the long term.12
Many ablation centres have long performed, and still do perform, PVI lesions with conventional low-power long-duration (LPLD) generator settings, aiming to achieve transmural ablation lesions while reducing the risk of steam pops, cardiac tamponade, PV stenosis and other complications. Conventional settings at most ablation centres are usually in the range of 25–40 W for 20–40 s at each lesion site, with a CF range of 10–20 g.13–15 Significant rates of PV reconnection with additional long procedure times when using these settings have prompted recent research in the use of high-power short-duration (HPSD) ablation. However, the definition of HPSD ablation is arbitrary and shows considerable variability in the literature, ranging from 50 W to 90 W with varying durations in the different HPSD studies.15–17 The safety and efficacy of HPSD ablation, as well as its effectiveness as an alternative treatment strategy are nevertheless the focus of ongoing research. For the purpose of this review article, high power is defined as the use of an ablation power output equal to or above 50 W.
Biophysics of radiofrequency lesion formation
During RF ablation, electromagnetic energy is transformed to thermal energy, resulting in tissue heating, irreversible cell injury and ultimately coagulative necrosis. This occurs via two consecutive mechanisms: resistive (direct) and conductive (indirect) heating (Figure 1).15 Resistive heating (also known as ‘Ohmic heating’) results from the principle that the electric current passage through a conductor produces heat. This is a rapid active process, beginning and ceasing immediately with the initiation and discontinuation of RF energy delivery.15,18,19 During the resistive phase, electrical current is delivered at the catheter–tissue interface and within the myocardium itself, leading to immediate heating of the superficial tissue layer (approximately 1–2 mm).
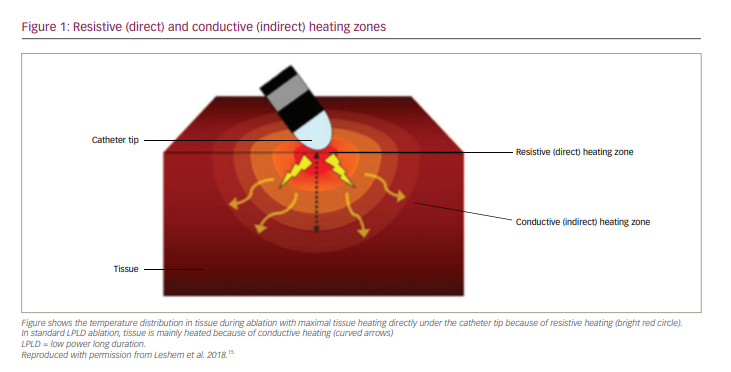
The resistive heating phase creates a heat source that then extends passively to deeper tissue layers during the conductive phase. This thermal conduction follows the principle that heat is conducted along temperature gradients. Conductive heating is a passive process, as heat is transferred away from the ablation lesion core. It is a time-dependent process, resulting from the current applied and heat produced in the resistive phase, and takes from seconds to a couple of minutes to reach a stable state of thermal equilibrium.15,18,19 The standard RF lesion using low-to-moderate power settings and a relatively long duration (LPLD ablation) is created primarily by conductive heating.
Biophysics and animal studies in high-power short-duration ablation
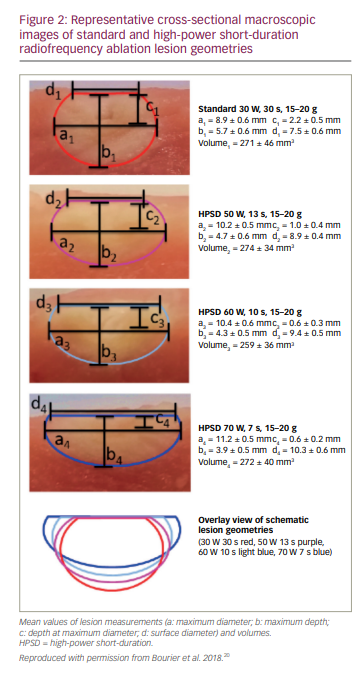
Contrary to LPLD ablation, HPSD ablation creates a larger zone of direct resistive heating of the tissue. Thus, the ratio of resistive to conductive tissue heating increases, with irreversible injury occurring mainly in the resistive zone of the endocardium. The lesion geometry in HPSD ablation is also altered due to this change in ratio between resistive and conductive heating. Lesion diameters of HPSD applications are significantly larger, whereas their lesion depths are significantly smaller when compared with standard settings.15,20 Shorter energy delivery of HPSD results in reduced temperature rise in deeper tissue, resulting in reduced depths of HPSD lesions when compared with standard lesions. The lesion width of HPSD ablation is maximal endocardially as well as subendocardially, whereas the LPLD lesion width is maximal in the subendocardium.
In an ex vivo model (porcine thigh muscle), it was shown that standard settings (30 W, 30 s, 15–20 g) produce a more spherical lesion, with a mean depth of 6 mm and maximal diameter deeper in the tissue, whereas HPSD ablation (60 W, 10 s, 15–20 g or 70 W, 7 s, 15–20 g) produces a more oval shallow lesion (mean depth 4 mm), with a larger maximal diameter located in the endocardium (i.e. produces wider and flatter lesions).20 Lesions with HPSD ablation, however, have similar lesion volumes as standard ablation (Figure 2).20 In another in vitro model and a sheep model, several combinations of high-power settings were studied: a wide range of energy settings, from 50 W to 80 W for 5 s, while using a temperature cut-off of 60°C and an ablation catheter with CF sensing. The HPSD ablation settings resulted in 100% transmural lesions while reducing collateral damage compared with ablation power at 40 W for a duration of 30 s in the right atrium.21
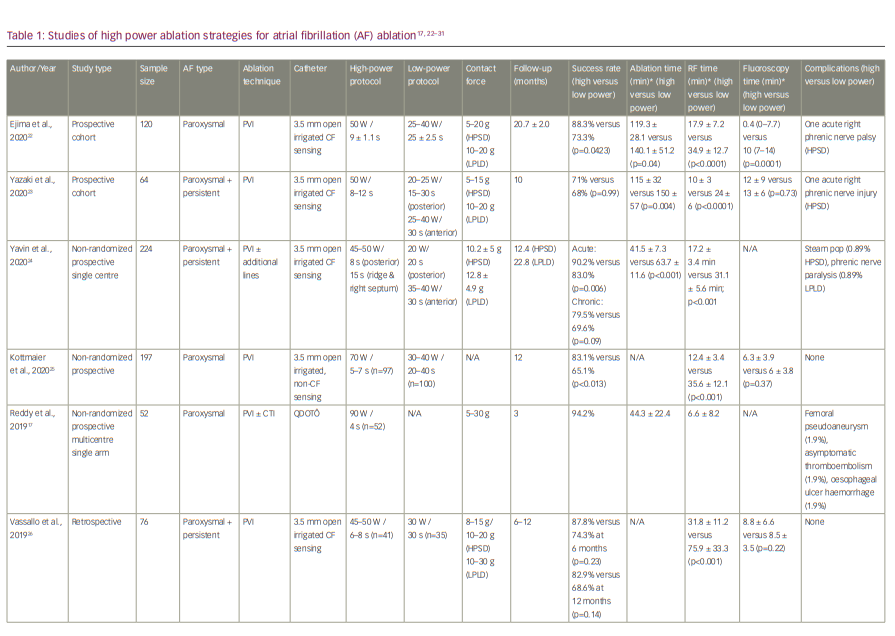
High-power short-duration ablation in human subjects
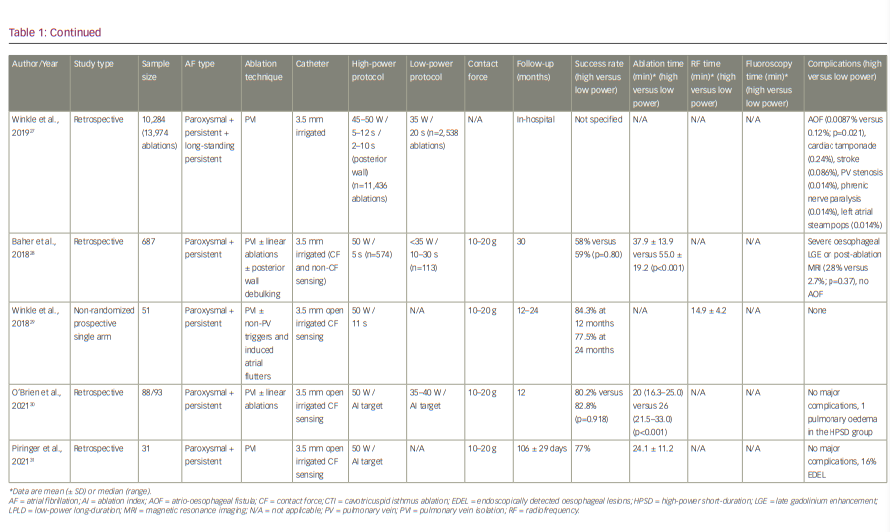
The shallower but wider lesions with less endocardial sparing that are generated with the implementation of HPSD ablation may be favourable for thin-walled tissues such as the atria, where contiguity is important for the efficacy, whereas reduced lesion depth can potentially reduce collateral injury to neighbouring anatomical structures such as the oesophagus, predominantly due to the reduction of the conductive heating phase. Therefore, several studies have already been conducted in humans to assess the safety and efficacy of the HPSD ablation strategy. There is significant heterogeneity in the settings/power, types of ablation catheters and duration of follow-up in the different studies. As CF-sensing catheters are now the mainstay of AF ablation, and CF measurement is essentially imperative in implementing an HPSD ablation strategy, Table 1 summarizes HPSD ablation studies performed after the introduction of CF-sensing catheters.17,22–31 In a study by Reddy et al., the concept of very-high-power short-duration (vHPSD) ablation for PVI was studied in a multicentre setting.17 A newly designed CF catheter (QDOT Micro™ catheter; Biosense Webster, Inc., Irvine, CA, USA), which was used to deliver 90 W/4 s lesions, enables real-time temperature monitoring using microelectrodes and six thermocouples. The vHPSD algorithm automatically adapts the power applied, using the information of the hottest surface thermocouple (cut-off point at 65–70°C), which reduces excessive heating of cardiac tissue and in turn the risk of a steam pop.
High-power short-duration ablation:
Procedure efficiency and outcomes
Table 1 demonstrates that an HPSD ablation strategy is associated with shorter ablation times and RF times versus an LPLD strategy.17,22–28 Most studies also showed significantly lower fluoroscopy time with HPSD compared with the LPLD technique, although not always reaching statistical significance.22,23,25,26,30 Reduction in procedure time is not only an advantage for the operator, but also beneficial for the patient, as longer ablation times can lead to fluid overload from catheter irrigation and as a result have been associated with worsening heart failure in patients with left ventricular systolic dysfunction.32 An increased post-procedural cognitive dysfunction has also been described with longer ablation times.33
Most human studies also demonstrated that an HPSD ablation strategy is associated with favourable outcomes and a better long-term freedom from AF and other atrial arrhythmias compared with the LPLD technique. In the study by Kottmaier et al., the HPSD group (70 W/5–7 s) showed significantly less arrhythmia recurrence during 1-year follow-up, with 83.1% of patients free from AF compared with 65.1% in the standard group (p<0.013).25 The recent prospective study by Yavin et al. investigated HPSD by comparing 112 patients who underwent HPSD ablation with 112 historical controls who underwent standard ablation.24 This study found a higher acute success rate with HPSD ablation (90.2% versus 83.0%; p=0.006), in addition to a shorter RF time (17.2 ± 3.4 min versus 31.1 ± 5.6 min; p<0.001). The incidence of chronic PV reconnection was also lower with HPSD ablation (16.6% versus 52.2%; p=0.03), whereby areas of chronic reconnection were associated with catheter motion ≥1 mm for ≥50% of the application duration. In a higher proportion of HPSD applications, catheter motion was <1 mm during ≥50% of the application duration (88.6% versus 72.8%; p<0.001), allowing energy delivery with greater stability. In a very recent study by O’Brien et al., ablation index (AI)-guided HPSD AF ablation also proved to be as safe and effective as LPLD, and resulted in reduced ablation and procedure times.30 RF times (20 min versus 26 min; p<0.001) and total procedure times (130 min versus 156 min; p=0.002) were significantly lower in the 50 W HPSD group, whereas 12-month freedom from arrhythmia was similar in both groups (80.2% versus 82.8%; p=0.918).
These findings highlight the importance of catheter stability and sufficient CF at every lesion location for a durable lesion formation. The absolute time required for catheter stability is shorter with HPSD ablation, leading to energy delivery with greater stability. Therefore, it is not recommended to implement a ‘dragging’ technique with HPSD ablation, as the possibility of catheter instability and time with insufficient contact would increase, leading in turn to tissue oedema rather than an efficient ablation lesion. In the QDOT-FAST trial,17 the use of 90 W/4 s and a thermocouple temperature cut-off at 65–70°C was associated with PVI in all 52 patients acutely, with a success rate of 94.2% at 3 months. These findings emphasize the equal importance of power as a pivotal parameter in the quality and durability of each lesion. Commonly used real-time surrogates for durable lesion formation, which take power into account such as lesion index (LSI) and AI, can be used in most HPSD ablation scenarios to increase lesion quality.30,34,35 However, an uncritical chasing of a targeted AI/LSI value can possibly lead to overheating, and thus many centres use specific, though arbitrary, time limits for HSPD in order to avoid some complications.
Recently published meta-analyses comparing HPSD with LPLD RF PVI strategies confirmed similar or better procedural effectiveness with the HPSD approach when compared with conventional RF ablation, with comparable safety and shorter procedural duration.36,37 In a meta-analysis by Ravi et al.,36 there was no difference in total complications between the two groups, whereas total procedure duration (mean difference -37.35 min; p<0.001), fluoroscopy duration (mean difference -5.23 min; p=0.001) and RF ablation time (mean difference -16.26 min; p<0.001) were all significantly lower in HPSD PVI. HPSD PVI also exhibited higher freedom from atrial arrhythmia in the subgroup analysis of patients with paroxysmal AF (odds ratio [OR] 1.80, 95% confidence interval [CI] 1.29–2.50; p<0.001), in studies with a ≥50 W protocol in the HPSD group (OR 1.53, 95% CI 1.08–2.18; p=0.02) and in studies with CF sensing catheter use (OR 1.65, 95% CI 1.21–2.25; p=0.002). On the other hand, in a meta-analysis by Kewcharoen et al.,37 an HPSD strategy was marginally not associated with increased freedom from atrial tachyarrhythmia at 12-month follow-up (OR 1.54, 95% CI 0.99–2.40; p=0.054) and exhibited similar serious complications compared with an LPLD approach. However, there was a significant reduction in the HPSD group for total procedure time (weighted mean difference 49.60, 95% CI 29.76–69.44) and ablation time (weighted mean difference 17.92, 95% CI 13.63–22.22), but not for fluoroscopy time (weighted mean difference 1.15, 95% CI -0.67–2.97).
Complications
Major complications occur in approximately 3.5% of AF ablation procedures because of direct and collateral injury, among other factors.38,39 While there is vast worldwide experience with conventional ablation settings, the safety profile of HPSD ablation is much less studied. A single retrospective study of almost 14,000 ablations demonstrated low complication rates, but procedure technique, ablation catheter and technology varied, with a duration of application in the range of 2–15 seconds.27 In the study by Kottmaier et al. a power-controlled ablation strategy of 70 W/7 s at the anterior and 70 W/5 s at the posterior left atrium was compared with a conventional protocol of 30–40 W/20–40 s using a standard irrigated ablation catheter, without, however, CF measurement.25 A Flexability SE catheter (Abbott, Minneapolis, MN, USA) was used for PVI in this study, with a tip design that leads to significantly fewer steam pops compared with other ablation catheters.25 The authors reported no complications in any of the 197 patients who participated in the study, although no oesophageal temperature monitoring was used and no endoscopy was performed to check for endoscopically detected oesophageal lesions. In a recent trial by Piringer et al., 31 patients underwent first-time AF ablation (PVI) with an HPSD ablation technique (50 W, with a target AI of 350 at the posterior wall).31 In this patient population, an endoscopically detected oesophageal lesion was confirmed in 5 of 31 patients (16%).
Another study investigated late gadolinium enhancement magnetic resonance imaging of the oesophagus in 574 patients following AF ablation using user-defined HPSD settings of 50 W for 5 s.28 The authors reported a 14.3% incidence of moderate-to-severe thermal oesophageal late gadolinium enhancement, although no fistulas were reported. However, the incidence of atrio-oesophageal fistula is generally low (1/2,000 to 1/4,000) and thus most studies are underpowered to detect such a devastating complication.
In the QDOT-FAST trial, despite a small cohort, one patient developed a haemorrhage from an oesophageal ulcer, which was diagnosed 1 day after the ablation and was treated medically.17 However, there was no incident of death, stroke, atrio-oesophageal fistula or PV stenosis. Furthermore, there have recently been reports of a higher than anticipated number of char formations on the ablation catheter tip in hospitals using the nGEN generator and the QDOT Micro™ catheter in the QMODE+ setting. Thus, more data are needed for vHPSD ablation regarding safety and possible occurrence of thromboembolic events.
Other new/evolving ablation techniques
Pulsed‐field ablation (PFA) has recently emerged as an effective, safe and promising non-thermal alternative to RF ablation for the treatment of AF. Ablation can be achieved by employing high-voltage biphasic direct-current pulses with a duration of milliseconds delivered in a bipolar manner by multipolar single-shot catheters.40 This leads to the formation of permanent nanoscale pores in the cardiomyocyte membrane and thus myocyte death. This electroporation method creates transmural lesions in less than 1 s and has been shown to have high specificity for cardiac tissue, without affecting vascular or nervous tissue or oesophagus integrity.
The efficacy and safety of PFA was demonstrated in three consecutive non‐randomized trials with a total of 121 patients with paroxysmal AF.41,42 PVI was achieved within an ablation time of a few minutes, and freedom from atrial arrhythmias at 1 year was 78.5% in this population.
Reddy et al., also assessed the safety and efficacy of PFA in 25 patients with persistent AF.43 In addition to feasibility of posterior wall isolation, they demonstrated durable PVI in more than 90% of the PVs, with no signs of oesophageal damage despite PVI and extensive posterior wall ablation.
Although PFA has the potential to achieve very rapid ablation, reported total procedure times are still in the range of conventional RF ablation procedures (97 and 148 minutes, respectively) with relatively high fluoroscopy times, most probably reflecting the learning curve of the novel method.42 The long-term safety, efficacy and effectiveness of PFA remain unknown, and there are currently no randomized data comparing PFA and HPSD strategies for PVI.
Conclusions
The HPSD ablation strategy is a promising alternative approach to RF energy delivery, potentially contributing to a superior, more durable lesion formation, shorter procedural and RF times, and similar complication rates compared with LPLD ablation. The optimum duration and power of energy application, however, have yet to be established.
Large clinical trials are needed to assess not only the safety and long-term efficacy of HPSD ablation, but also novel ablation catheters that can deliver very high power at extremely short duration, to validate the use of this strategy in different populations and clinical settings, before advocating its widespread use in AF ablation. Furthermore, randomized studies comparing the PFA and HPSD approaches could shed light on the comparative advantages and disadvantages of these parallel evolving ablation strategies, with the ultimate goal of improving the risk–benefit ratio in AF ablation.