Introduction: Shockable ventricular arrhythmias account for one third of all cardiovascular deaths worldwide. Several randomised control trials have demonstrated ICD and CRT-D devices reduce mortality however the primary concern for both patient and physician remain inappropriate shocks. Aside from the pain and psychological impact, inappropriate shocks have also been independently associated with mortality in patient’s recipient of an ICD. To the authors knowledge, there are currently no published UK observational studies in real-world clinical practice.
Methods: The study population consisted of 160 patients with ICD or CRT-D implants who were under regular review between 2011-2019 at the pacing clinic at Hereford County Hospital, UK. The appropriateness of any delivered shocks or ATP was determined by physiologist review of the device intracardiac electrograms, with adjudication from a consultant cardiologist as required. Electronic health records were reviewed.
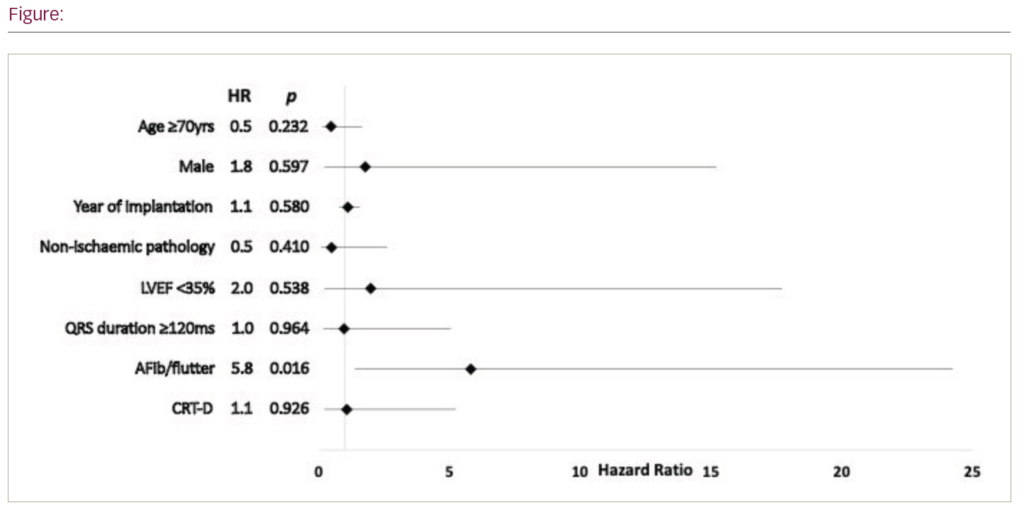
Results: During the study period 93 ICD (68 single- and 23 dual-chambered) and 67 CRT-D recipients were under regular review for a median follow-up duration of 3.5 years (IQR: 2.2 – 5.1). Mean age at time of device implantation was 68 yrs; 87 patients (54%) were aged 70 yrs or over, and 122 (76%) were male. Pre-device cardiac status was as follows: 112 (73%) had an LV ejection fraction <35%, 104 (74%) had ischaemic pathology and QRS duration was <120 ms in 64 (51%). 50 (34%) patients had existing atrial fibrillation (AFib). Optimal target dose pharmacotherapy was achieved as follows: ACEI/ARB – 65 (47%), b-blocker/ivabradine – 64 (43%), MRA – 75 (58%). 11 (6.9%) patients experienced a total of 18 inappropriate shocks and AFib/flutter was the trigger in 10 of these patients (91%). 6 (3.7%) patients experienced more than one inappropriate shock. The cumulative event rate for first inappropriate shock was 5% at 1 year and 11% at 5 years. Compared to patients receiving appropriate shocks, patients inappropriately shocked were more likely to have pre-device AFib (100% vs 33%, p=0.012) and non-ischaemic pathology (33% vs 0%, p=0.034), and were more likely to be on optimal target dose b-blocker/ivabradine (100% vs 31%, p=0.009). 23 (14%) patients died during follow-up. Cumulative mortality rate was 5% at 1 year and 21% at 5 years. Cox proportional hazards models demonstrated pre-device AFib was an independent predictor of inappropriate shocks, and electrical therapy (HR 5.8, p=0.016, Figure 1). Pre-device characteristics and prior delivery of shocks and ATP did not predict all-cause mortality in multivariate analyses.
Conclusions: This is the first UK study to document the real word burden of inappropriate shocks in unselected ICD and CRT-D recipients; it is lower than published European cohorts and lower than seminal clinical trials. AFib/flutter is an independent predictor of inappropriate shocks and electrical therapy. Stringent anti-arrhythmogenic strategies including consideration of catheter ablation are required in this cohort.