Introduction: Cryptogenic stroke accounts for 30–40% of ischaemic strokes, and may be due to undetected atrial fibrillation (AF). Conventionally, 24-hour ECG monitoring is used to detect AF after a cryptogenic stroke; however, the CRYSTAL AF trial concluded that insertable cardiac monitoring (ICM) was superior to conventional-hour ECG monitoring in AF detection with 19.0% and 2.3% detection rate, respectively, at 3 years. NICE has now recommended the use of Reveal LINQ™ ICM (RL-ICM) as an option for detection of AF after cryptogenic stroke if initial Holter monitoring has failed to detect AF.
Methods: At Nottingham University Hospital NHS Trust, we established a service with the Stroke Team for RL-ICM insertion in patients with cryptogenic stroke for AF detection. The inclusion criteria were confirmation of stroke/transient ischaemic attack, sinus rhythm on 12-lead ECG, no embolic source on transthoracic echocardiogram, negative prolonged Holter monitoring, modified Rankin score 0–3, normal CT or MR angiogram of brain, normal carotid ultrasound, and normal hyper-coagulable screening if <55 years old. The exclusion criteria were known AF or atrial flutter, indication for pacemaker or implantable cardiac defibrillator, anticoagulation deemed inappropriate, or already on anticoagulation.
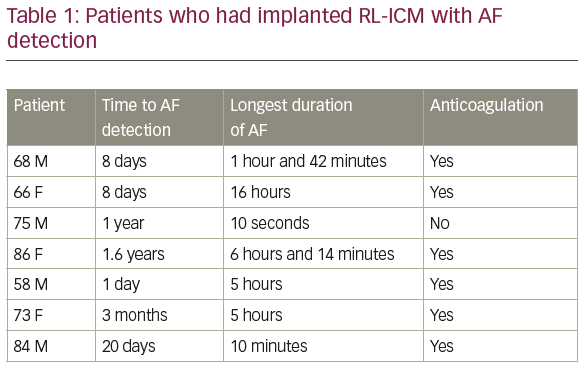
Results: Between 29th January 2018 and 6th May 2021, 55 patients were referred for RL-ICM insertion from the Stroke Team (age 63.4 ± 14.7 years; range 24–94); male 34 (61.8%); stroke 49 (89); transient ischaemic attack 6 (10.9%); hypertension 23 (41.8%); diabetes 7 (12.7%); hypercholesterolaemia 22 (40%); current smoker 2 (3.6%); and coronary artery disease 7 (12.7%). Twenty-five (45.5%) patients have been implanted to date, with implants dates ranging between 27th March 2018 and 27th May 2021. The average time between referral and RL-ICM implantation was 82 days (median 74 days; range 29–321). Atrial fibrillation has been detected in 7 (28%) patients. The mean time for detection was 153 days (median 20 days; range 1–579). The shortest episode of AF was 10 seconds and the longest was 16 hours with a mean of 3 hours. Once detection was established, out of the 7 patients, the Stroke Team started anticoagulation in 6 (85.7%) patients; 1 patient was not anti-coagulated due to his AF lasting <30 seconds. Two of these patients died; one due to COVID-19 pneumonia and the other of an unclear cause. No patient had further stroke while awaiting RL-ICM implantation.
Conclusion: The current detection rate of AF at our centre is 28% at a median time of 20 days. Although the number of patients in our cohort is very small, this appears to be much higher than the findings of CRYSTAL AF where detection rate of AF was 19% at 3 years. Our RL-ICM implant waiting times have significantly increased due to the COVID-19 pandemic, with currently 30 cryptogenic stroke patients still awaiting implantation. The initial high detection rate of AF in our patient population with RL-ICM highlights the importance of this service, and the need for finding solutions to our long waiting times for RL-ICM insertion.