Introduction: Cardiac implantable electronic devices (CIEDs) are a well-established treatment of cardiac arrhythmias, comprising pacemakers, implantable cardioverter defibrillators and cardiac resynchronisation therapy. In order to ensure continued reliable delivery of CIED therapy, it is essential patients receive high-quality device monitoring.1 This is mostly done with in-person reviews of symptoms, disease events and device function. However, newer CIEDs can communicate functional data remotely to provide early detection of disease events and battery depletion.2 While CIED battery depletion is gradual, it is often unpredictable, and ideally batteries must be replaced before the elective replacement indicator (ERI). At this time, the CIED reprograms to conserve battery with reduced functionality and thus negatively impacts patient experience.3 During the COVID-19 pandemic, capacity for in-person CIED follow-up at our centre was greatly reduced. We aimed to evaluate the impact this had on patient care by reviewing whether there was an increase in non-elective admissions for patients who had reached ERI and so needed a non-elective battery replacement (NEBR).
Methods: The records of patients with a CIED who underwent a battery change between March 2018 to March 2022 were retrospectively reviewed at our tertiary cardiac centre. We reviewed the electronic discharge summary of all patients with a length of stay over 24 hours to determine the reason for their admission.
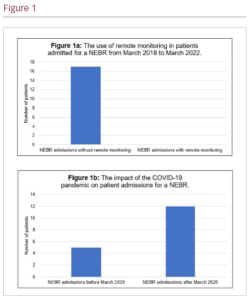
Results: A total of 485 patients underwent a CIED battery change in this 4-year period. Of these, 42 patients were admitted for over 24 hours. Overall, 17 patients were admitted for an NEBR after it was found their CIED had reached the ERI. Notably, none of these patients had been established on remote follow-up (Figure 1a). The mean length of hospital stay for patients admitted for an NEBR was 3 days and 10 hours. Two patients had an extended length of stay due to a hospital-acquired infection. In the 2 years before the COVID-19 Pandemic, March 2018 – March 2020, 5 patients (2.1%) were admitted for an NEBR. In the period March 2020 – March 2022, 12 patients (4.7%) were admitted for an NEBR (p=0.13) (Figure 1b).
Conclusion: Following the COVID-19 pandemic, twice as many patients required admission for an NEBR. No patients who required an NEBR had been established on remote follow-up. Remote monitoring can be programmed to alert healthcare providers of CIED issues including battery longevity, lead integrity, arrhythmias and large changes in electrical parameters. Greater provision of remote monitoring may reduce the risk of patients requiring an NEBR. ❑