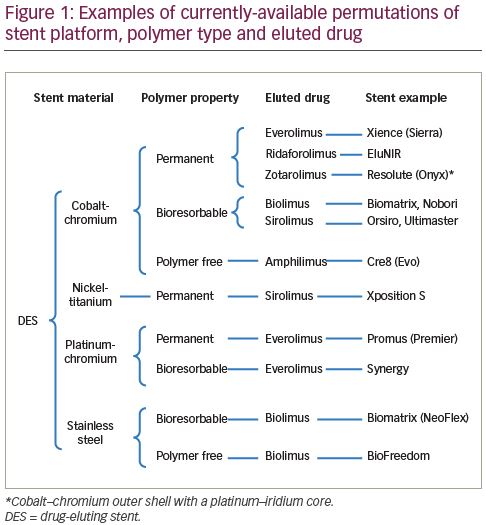
Coronary artery disease (CAD), due to development of atherosclerotic plaques, remains a leading cause of morbidity and mortality. If unstable or clinically significant, these are frequently treated with percutaneous coronary intervention (PCI), typically involving balloon angioplasty and implantation of a metallic drug-eluting stent (DES).1 Coronary stents have substantially evolved since their first use in the 1980s due to continuous work to refine their design, structure and materials. A number of DESs, differing in design and composition, are currently available for clinical use and are summarised in Figure 1. The manufacture and sale of DESs has become a large industry, with the market expected to be worth up to 17 billion US dollars by 2025.2 In 2013, >75% of the global DES market share (sampled in the 10 major markets) was held by three companies: Abbott Vascular (Chicago, IL, USA; manufacturer of, for example, XIENCE® stents), Boston Scientific (Marlborough, MA, USA; e.g., Promus®) and Medtronic (Dublin, Ireland; e.g., Resolute®). The market continues to develop, with particular expansion occurring in Asia and more expected in the coming years.3 Emerging manufacturers, for example in China, are predicted to challenge current dominance.4 The choice of a stent for a particular patient and coronary lesion, however, largely remains the operator’s choice. This article reviews the structure, design and clinical data of commonly-used contemporary DESs to identify the differences and whether any particular stent performs better than others.
Evolution of drug-eluting stents
Early years of angioplasty and bare metal stents
Coronary angioplasty was first performed by Andreas Gruentzig in 1977.5 Initially, angioplasty was performed without stent deployment; however, its efficacy was reduced by acute closure or re-stenosis. Coronary stents were developed to overcome these shortcomings.6 The vast majority of PCI procedures performed currently involve angioplasty with stent deployment. Wallstent™, (Schneider AG, Bulach, Switzerland), a ‘self-expanding, elastic, macroporous tubular prosthesis, woven from stainless steel’, was the first stent implanted in a human coronary artery in 1984 by Sigwart and colleagues.7 In 1987, Schatz and co-workers developed the first stent to obtain FDA approval (Palmaz-Schatz®; Johnson & Johnson, New Brunswick, NJ, USA). This was the first balloon-expandable, stainless-steel, tubular stent, and was widely used throughout the 1990s. In the early years of that decade, a number of other stents became available, including FlexStent® (Cook, Bloomington, IN, USA), Wiktor® (Medtronic, Minneapolis, MN, USA), Micro® (Applied Vascular Engineering, Twickenham, UK), Cordis® (Cordis, Santa Clara, CA, USA), and MULTI-LINK® (Advanced Cardiovascular Systems, Santa Clara, CA, USA).6
These bare metal stents (BMSs) reduced acute vessel closure and early elastic recoil; however, they were bulky, difficult to use and associated with frequent device failure. Furthermore, the risk of in-stent restenosis was significant due to proliferation and migration of vascular smooth muscle cells within the device.8 The use of stents, therefore, remained limited to cases of acute/threatened closure or restenosis after balloon angioplasty until two landmark trials, the Belgium Netherlands Stent Arterial Revascularization Therapies Study (BENESTENT) and the North American Stent Restenosis Study (STRESS), showed superiority of BMSs over balloon angioplasty.9,10 However, the incidence of in-stent restenosis remained as high as 20–30%.
Development of drug-eluting stents
Coating stents with anti-proliferative drugs, e.g., sirolimus or paclitaxel, substantially reduced in-stent restenosis.11,12 When incorporated within a polymer and coated on the surface of BMSs, these were released slowly over a few weeks after deployment. Eduardo Sousa implanted the first sirolimus-eluting stent in 1999 and it became available for clinical use as the CYPHER™ (Cordis) stent in 2002. CYPHER was tested in numerous randomised controlled trials (RCTs), demonstrating a significant reduction in in-stent restenosis and target vessel revascularisation (TVR) compared with BMSs.11 TAXUS® (Boston Scientific), a paclitaxel-eluting stent, closely followed CYPHER, and again many RCTs (TAXUS I-IV) confirmed its efficacy against BMSs.12,13 Early-generation DESs, however, were linked with risk of stent thrombosis, possibly due to delayed endothelialisation by the anti-restenotic drugs or delayed hypersensitivity reaction to the polymer in DESs.14,15 The concerns of stent thrombosis with early-generation DESs stimulated development of novel anti-platelet agents, better polymers, and newer generation of DESs.
Components of a drug-eluting stent
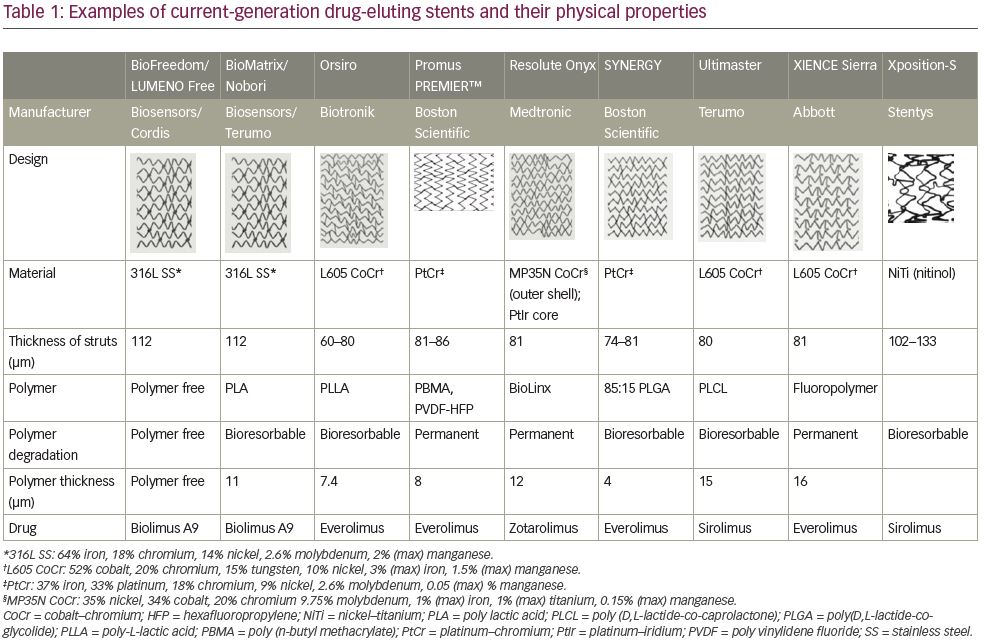
There are three major components of a DES: the metallic platform, polymer (if present) and anti-proliferative drug. Examples and properties of currently-available stents with different permutations of this construction are shown in Table 1.
Metallic platform
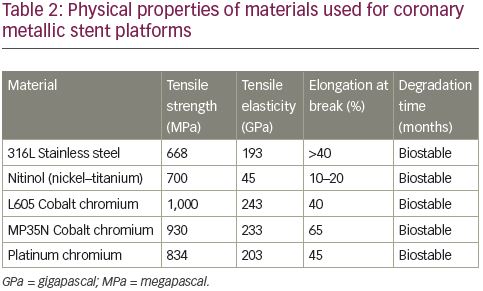
First-generation stents were typically made of stainless steel as it provides adequate radial strength to restore the patency of a stenotic artery. Although stainless steel remains a material used in some current-generation DESs (e.g., BioFreedom™, BioMatrix™ [Biosensors International, Singapore], Nobori® [Terumo, Tokyo, Japan]), alloys are more commonly employed. These include cobalt–chromium (e.g., XIENCE and Ultimaster™ [Terumo]), platinum–chromium (e.g., Promus and SYNERGY™ [Boston Scientific]) and nickel–titanium (also known as nitinol e.g., Xposition [Stentys, Paris, France]). Some stents include two alloys, for example the Resolute Onyx™ (Medtronic) stent has an outer shell of a cobalt-based alloy but a platinum-iridium core in order to increase radiopacity.16 Physical properties of commonly-used materials for metallic DES platforms are summarised in Table 2. Compared to stainless steel, these alloys have intrinsically greater tensile strength whilst possessing similar or reduced elasticity. This allows manufacturing of thinner struts (typically >100 µm for stainless steel versus <100 µm for alloys) whilst maintaining strength, improving flexibility, deliverability and reducing risk of stent thrombosis. Strut thickness and stent flexibility have an impact on the degree of injury, risk of rupture of the elastic laminae and overall inflammation, which may increase the risk of stent thrombosis events post-implantation. In addition, first-generation DES studies showed that thicker struts were associated with a higher incidence of side branch occlusion compared with thinner-strut DESs.17 Moreover, thinner-strut devices have been associated with better clinical outcomes.18
Polymers
In first-generation DESs, the drug was incorporated in permanent (i.e., non-biodegradable) synthetic polymers such as polyethylene-co-vinyl acetate, poly-n-butyl methacrylate, and the tri-block copolymer poly(styrene-b-isobutylene-b-styrene). Histopathological data indicated that the delayed vascular healing due to these polymer coatings may be associated with an increased risk of very late stent thrombosis after first-generation DES implantation.6 These polymers were superseded by more biocompatible permanent polymers (PPs) such as phosphorylcholine and a co-polymer of poly-vinylidene fluoride and hexafluoropropylene. Further development of DESs has led to those with biodegradable polymers, the rationale for these being that they behave as a conventional DES in the early phase and, once all the drug has been released and the polymer degraded, revert to a BMS as only the metallic platform remains. These typically utilise polylactic acid or a variation thereof as the polymer, which is broken down by hydrolysis over a period of months to lactic acid. Most recently, polymer-free DESs have come onto the market. Instead of utilising a polymer to contain and release the drug, this is directly applied to the metallic surface of the DES in micropores. These have the obvious theoretical advantage of avoiding polymer-related complications altogether.
Anti-proliferative drugs
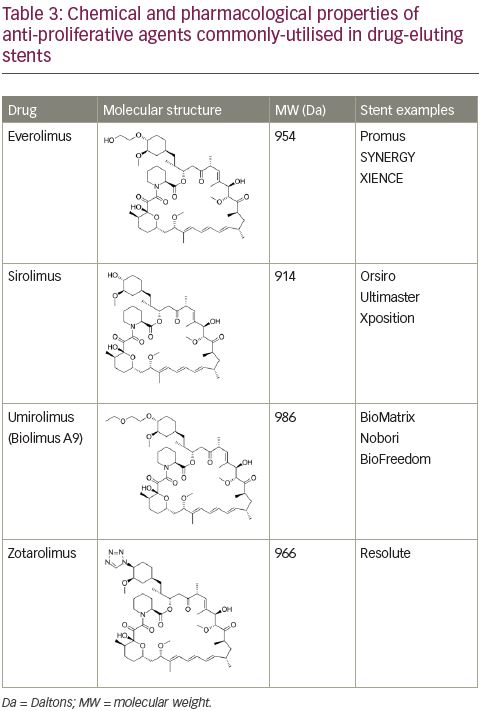
Amongst a variety of immunosuppressive and anti-proliferative agents tested to-date, mammalian target of rapamycin inhibitors have prevailed. Contemporary stents are mostly sirolimus-eluting stents, everolimus-eluting stents, zotarolimus-eluting stents or biolimus-eluting stents. Characteristics of these four commonly-used anti-proliferative drugs are shown in Table 3. However, the search for a better drug continues, and other drugs, including novolimus and myolimus, are being tested.19
Comparative data for contemporary drug-eluting stents
Stent platform and strut thickness
Cobalt–chromium and platinum–chromium stents have been tested against current-generation stainless-steel stents. The COMPARE-II trial examined the Nobori stainless-steel biolimus-eluting stent against the XIENCE cobalt–chromium everolimus-eluting stent and the Promus platinum–chromium everolimus-eluting stent. There were no significant differences in the primary composite endpoint of safety (cardiac death and non-fatal myocardial infarction [MI]) and efficacy (clinically indicated TVR) at 12 months.20 Similarly, no differences in clinical outcomes were seen in the NEXT trial comparing Nobori versus XIENCE/Promus.21 After 5 years, the stent thrombosis rate was 0.49% versus 0.34% for the Nobori and the XIENCE/Promus, respectively (p=0.52).22
Current generations of stainless-steel stents have also been tested against a reference group receiving only platinum–chromium stents. For example, in the SORT-OUT VIII trial, the BioMatrix stainless-steel biolimus-eluting stent was compared with the SYNERGY platinum–chromium everolimus-eluting stent. The primary endpoint of target lesion failure (TLF) at 1 year occurred in 4.0% (platinum–chromium) versus 4.4% (stainless steel), (non-inferiority p<0.001).23 The EVERBIO-II trial compared 80 patients implanted with the platinum–chromium everolimus-eluting stent (PROMUS) and 80 with BioMatrix stents and found no difference in late loss at 9 months (platinum–chromium 0.24 ± 0.32 mm versus stainless steel 0.25 ± 0.41).24
Platinum–chromium stents have similarly been assessed against cobalt–chromium stents. For example, the PLATINUM programme of studies tested Promus stents (platinum–chromium everolimus-eluting stents) against XIENCE stents (cobalt–chromium everolimus-eluting stents). In the PLATINUM study, which excluded those undergoing PCI for MI, TLF at 5 years was 9.1% versus 9.3% (hazard ratio [HR] 0.97, p=0.87). Similarly, there was little difference in stent thrombosis rates (0.8% versus 0.7%, p=0.79).25 PLATINUM PLUS, which was an all-comers study, showed that target vessel failure was 4.6% versus 3.2% (non-inferiority p=0.012, superiority p=0.08).26 Although there were no significant differences in the rates of cardiac death, ischaemia-driven target lesion revascularisation or MI, there did appear to be a nominal difference in the latter (1.6% versus 0.8%, p=0.09).
Stent mal-expansion and mal-apposition can be another problem with balloon-expandable stents.27 Nickel–titanium platform has been used to manufacture self-apposing stents, for example, the Xposition-S (Stentys). This facilitates better contouring of the stent to the vessel wall, especially useful in the tapering vessels. Earlier studies have been promising but further trials are warranted to define the use of this platform amongst current-generation DESs.28
Given that the main advantage of alloys over stainless steel is in allowing thinner struts whilst maintaining structural integrity, other analyses have sought to determine whether strut thickness itself has a significant effect on outcomes. Recent meta-analyses have demonstrated the benefits of devices with reduced strut thickness. Iantorno and colleagues combined the results of 69 trials (n=80,885) and divided these into ultrathin, thin, intermediate and thick strut devices. Compared to thick-strut DESs, those with ultrathin struts had significantly less stent thrombosis and MI (odds ratio [OR] 0.43, 95% confidence interval [CI] 0.27–0.68; and OR 0.73, 95% CI 0.62–0.92, respectively).18 This was consistent with another, smaller, meta-analysis of 10 trials (n=11,658), focusing on newer-generation stents and demonstrating that ultrathin strut DESs were associated with lower rates of stent thrombosis (risk ratio [RR] 0.72, 95% CI 0.51–1.01).29 Hence, it would be reasonable to conclude that stents with thinner struts (i.e., those made with cobalt–chromium or platinum–chromium alloys) are better than those with thicker struts, but that within current-generation DESs, choice of material itself does not appear to be a factor in determining clinical outcomes.
Polymers
Several studies have evaluated current-generation DESs with PP versus bioresorbable polymer (BP) coatings (Table 4).20,21,30–8 Whilst powered for, and typically demonstrating, non-inferiority, these studies have generally failed to show superiority of one stent type over another. Of note, however, the BIOFLOW-V trial, which compared a cobalt–chromium BP sirolimus-eluting stent (Orsiro®, Biotronik, Berlin, Germany) against a cobalt–chromium PP everolimus-eluting stent (XIENCE), demonstrated superiority in terms of TLF at 12 months, driven by a lower rate of target vessel MI (5% versus 8%, p=0.016).30 However, a direct comparison of a platinum–chromium everolimus-eluting stent with BP (SYNERGY) or PP (Promus) in the EVOLVE-II study showed non-inferiority, but no evidence of superiority and in fact, the rate of TLF at 12 months was numerically greater in the BP group.35
On the other hand, the SORT-OUT V trial, comparing stainless-steel biolimus-eluting stent with BP (Nobori) versus a first-generation stainless-steel sirolimus-eluting stent with PP (CYPHER), narrowly failed to show non-inferiority in the pre-specified intention-to-treat analysis of the primary composite endpoint of safety (cardiac death, MI, definite stent thrombosis) and efficacy (TVR) at 9 months.37 Other studies of the Nobori versus PP non-stainless steel everolimus-eluting stent, whilst demonstrating non-inferiority, have shown a similar or nominally greater incidence of the study primary endpoints, and also of stent thrombosis, in those receiving the BP DES. This pattern was not replicated, however, in the SORT-OUT VI trial of the BioMatrix BP biolimus-eluting stent, of very similar design to Nobori, versus a cobalt–chromium zotarolimus-eluting stent with PP (Resolute) nor versus CYPHER in LEADERS, which suggested nominal, but not significant, advantages of the BioMatrix device.36,38
One could argue that given the cobalt–chromium/platinum–chromium DESs with BP have consistently shown non-inferiority or superiority to those with PP, and there may be a signal of disadvantage with regards to the stainless-steel BP DESs, that the former should be preferred over the latter. This may be due to differences in strut thickness (e.g., Nobori 112 µm, Orsiro 60–80 µm), although Nobori appeared to perform worst when tested against a stainless-steel PP sirolimus-eluting stent of even greater strut thickness.37
Within the BP DES group, a limited number of studies have compared these head-to-head. The SORT-OUT VII trial tested Orsiro versus Nobori and found no significant difference in the primary endpoint of cardiac death, target lesion-related MI or target lesion revascularisation within 1 year, but this was numerically higher in the Nobori arm (4.6% versus 3.8%) and was accompanied by a significantly greater incidence of definite stent thrombosis (1.2% versus 0.4%, p=0.03).39 The SORT-OUT VIII trial tested the platinum–chromium BP everolimus-eluting stent (SYNERGY) against the stainless-steel BP biolimus-eluting stent (BioMatrix) and found, once again, no significant difference in TLF but a numerical advantage of the SYNERGY device (4.0% versus 4.4%).23 These data could be interpreted to suggest that if using a BP DES, stainless-steel devices may have disadvantages over newer alloy BP DESs.
Helpfully, several meta-analyses addressing this issue have now been published. For example, El-Hayek et al. studied 16 RCTs including 19,886 patients who had received either BP DESs or newer variants of PP DESs, followed up for a mean of 26 months.40 They concluded there were no significant differences in TVR, stent thrombosis or other clinical outcomes between the two types. This finding was not affected by stent material, eluted drug or strut thickness. Furthermore, a landmark analysis, performed to study very-late stent thrombosis, which is hypothetically reduced by use of BP DESs over PP DESs, showed no significant difference (RR 0.87, 95% CI 0.49–1.53, p=0.62). Conversely, Kobayashi and colleagues pooled data from nine trials (n=4,458) but with a longer mean follow-up of 63 months.41 Comparing thin-strut BP DESs with DP DESs; although there was no discernible difference in TLF (OR 1.04, 95% CI 0.89–1.21), there was a nominally reduced rate of definite/probable stent thrombosis in the BP DES group (0.78, 95% CI 0.59–1.01). Overall, long-term outcomes do not appear to be different between current DESs with DP and BP properties, likely due to improved biocompatibility of polymers in current-generation DESs.
Considering the polymer-free DESs currently available, there are limited data from RCTs comparing these with other DESs. A small study (NEXT, n=323) with angiographic outcomes, showed that the cobalt–chromium polymer-free sirolimus-eluting stent (Cre8™, Alvimedica, Istanbul, Turkey) offered reduced in-stent late lumen loss at 6 months when compared to a first-generation stainless-steel paclitaxel-eluting PP DES (TAXUS) (0.14 ± 0.36 mm versus 0.34 ± 0.40, p<0.0001 for non-inferiority and superiority); however, marked difference in strut thickness is a notable confounder.21 When compared to BMSs in patients at high-risk of bleeding in the LEADERS FREE trial, at 390 days the stainless-steel polymer-free BioFreedom biolimus-eluting stent was superior in the primary safety (composite of cardiac death, MI, or stent thrombosis) and efficacy (target lesion revascularisation) endpoints (9.4% versus 12.9% [HR 0.71, 95% CI 0.56–0.91, non-inferior p<0.001, superior p=0.005] and 5.1% versus 9.8% [HR 0.50 95% CI 0.37–0.69, p<0.001], respectively).42 There was no difference in rates of definite stent thrombosis between the groups (1.3% versus 1.4%, HR 0.93, 95% CI 0.47–1.84, p=0.84). These findings have recently been replicated in the LEADERS FREE II trial.43
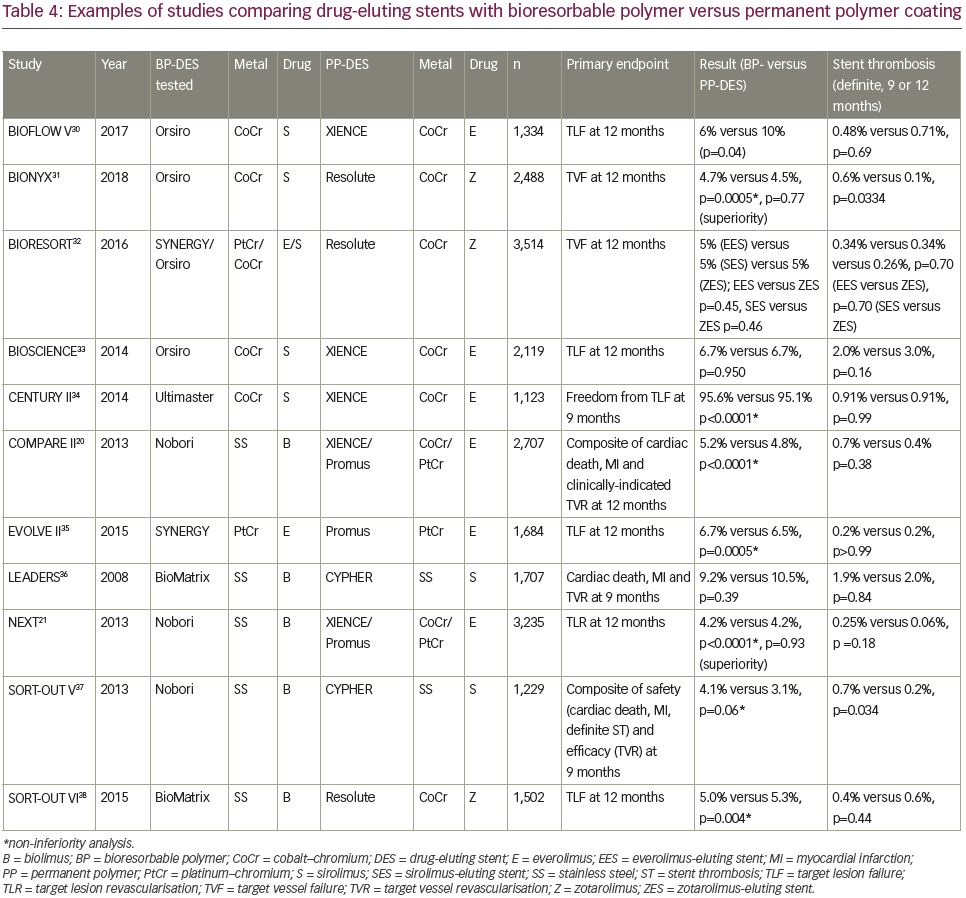
Although there have been no large RCTs comparing different types of polymer-free DES head-to-head, Chiarito and colleagues have recently published propensity-matched data obtained from the RUDI-FREE and ASTUTE registries assessing outcomes in those receiving two polymer-free DESs: the stainless-steel biolimus-eluting stent BioFreedom and the cobalt–chromium sirolimus-eluting stent Cre8. They found no significant differences in the primary endpoint of TLF at 12 months (4.0% versus 4.2%, HR 0.98, 95% CI 0.57–1.70, p=0.66), nor definite stent thrombosis (0.3% versus 0.7%, 0.49, 95% CI 0.10–2.45, p=0.31).44
Comparison of eluted drugs
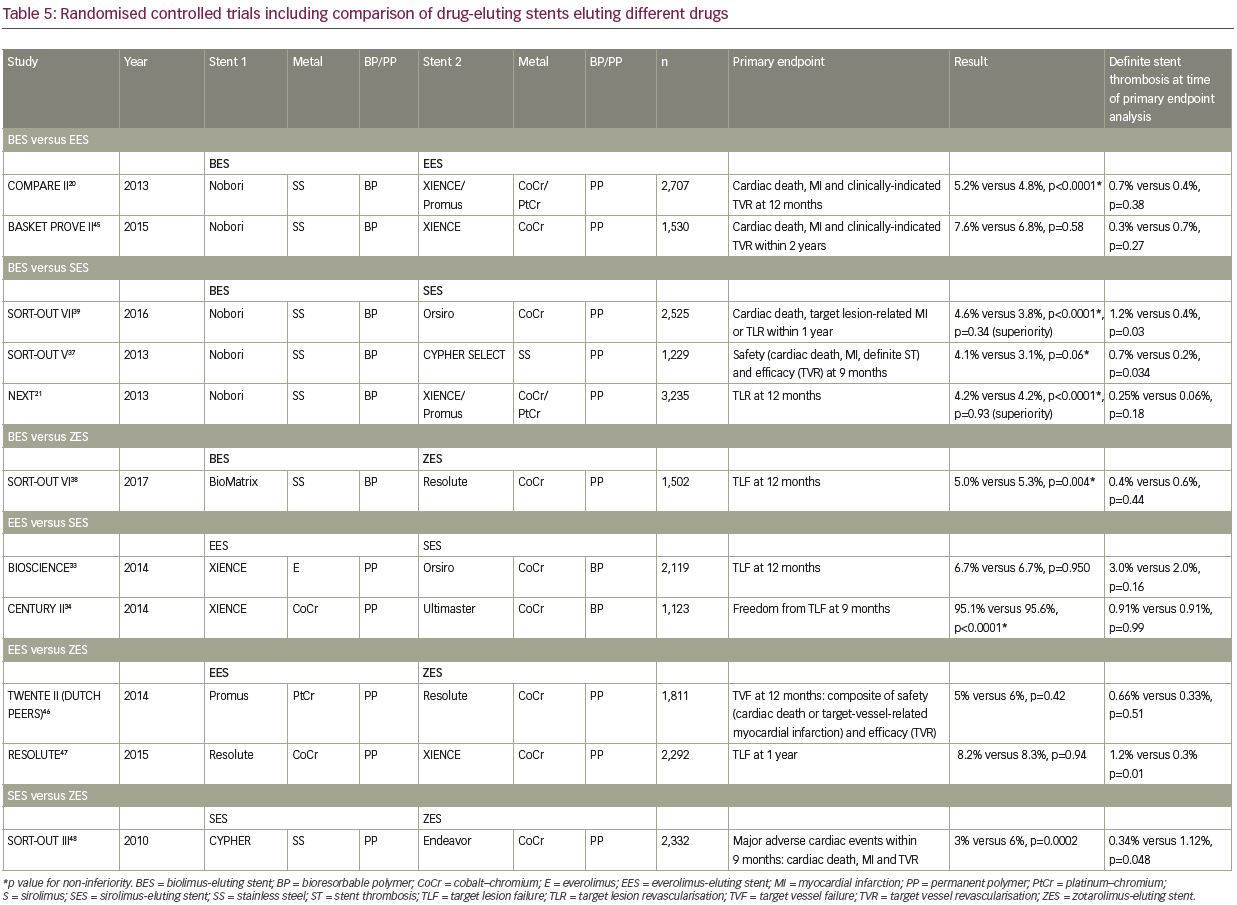
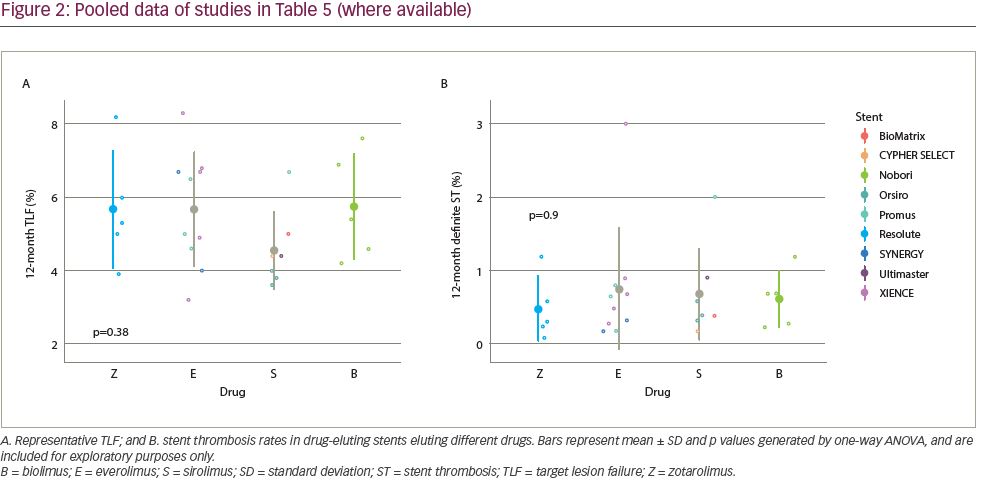
A summary of RCTs that compared stents eluting different drugs is shown in Table 520,21,33,34,37–9,45–8 and Figure 2. Piccolo and colleagues performed a meta-analysis of the Resolute PP zotarolimus-eluting stent against PP everolimus-eluting stent (Promus/XIENCE), including five trials and 9,899 patients.49 There were no discernible differences between the zotarolimus-eluting stents and the everolimus-eluting stents in rates of the studied outcomes, including TVR (RR 1.06, 95% CI 0.90–1.24, p=0.50) and definite or probable stent thrombosis (RR 1.26, 95% CI 0.86–1.85, p=0.24). There was also no difference in definite or probable very-late stent thrombosis (RR 1.06, 95% 0.53–2.11, p=0.87). Whilst these have not detected significant differences in the studied primary endpoints, some differences in stent thrombosis rates have been observed; for example, the Nobori stainless-steel BP biolimus-eluting stent was associated with a significantly greater risk of stent thrombosis compared to the stainless-steel PP sirolimus-eluting stent CYPHER SELECT™ and the cobalt–chromium BP sirolimus-eluting stent Orsiro in the SORT-OUT V and SORT-OUT VII trials, respectively (Table 5).37,39 However, it is difficult to discern whether this difference is due to the drug or other stent characteristics. Overall, one current-generation antiproliferative agent does not appear to have significantly different clinical properties to another.
Limitations of clinical data
Despite numerous clinical trials comparing DESs, these data have multiple limitations. Many trials are performed in selective groups of low-risk patients, often excluding elderly or unstable individuals with multiple comorbidities. Even ‘all-comer’ studies do not usually include all consecutive patients treated. Furthermore, it is usual for the statistical design of such studies to be based on proving non-inferiority over an established competitor, and although some pre-specify superiority analyses, the studies are not necessarily powered to detect this. The stents chosen for use in the active comparator arm of these studies often represent the older generations of DES or a BMS in situations where these have been regarded as standard-of-care. The primary endpoint of many trials is TLF, typically a composite of cardiac death, target-vessel-related MI, and clinically-indicated target lesion revascularisation (TVR) at 6, 9 or 12 months and clinical follow-up to 5 years. However, the endpoints likely to differentiate DESs, for example stent thrombosis, are rare, making it very difficult to conduct an adequately-powered study. Meta-analyses have helped to increase statistical power but have their own limitations. Additional difficulty in drawing conclusions comparing current DES performance arises from the fact that robust RCTs have not been performed to evaluate the very latest generation of stents in a ‘family’ (e.g. XIENCE Sierra, Promus PREMIER), and marketing authorisation has been granted on the basis of RCTs including the previous generations, combined with small-scale evaluation of the new design.
How to choose a stent for a patient
The availability of several DESs with similar data, whilst giving greater choice, may create confusion in selecting the optimal stent for a given patient. One may adopt a ‘one size fits all’ approach; however, there are subtle differences which may help to identify more suitable DESs for a particular patient or lesion type.
A workhorse stent
Data discussed above suggest that several current-generation DESs are perhaps comparable in both safety and efficacy. Any of these DESs can be used as a workhorse stent for the majority of patients. It would perhaps be ideal that at least two different DESs, with different characteristics and size matrix, are available in each cardiac catheterisation laboratory to make a more appropriate choice. The comparable safety and efficacy of DESs may also prompt different hospitals to largely determine their inventory of stents based on the cost of a DES. Depending on the volume of PCI cases in an institution, even a small difference in price may make significant impact on annual budget. It may also be an important factor in those healthcare systems where the patient or insurance has to pay.
Stents for specific patient groups
Most of the stent trials have generally shown no interaction between patient sub-groups and efficacy of one stent type over another. However, subtle differences have been observed. For patients with stent thrombosis elevation MI, perhaps DESs with BP are preferable.36,50 This has been further supported by the recently reported BIOSTEMI trial showing superiority of ultra-thin BP sirolimus-eluting stent Orsiro over DP everolimus-eluting stent XIENCE with respect to TLF at 1 year.51 For patients with high bleeding risk, BioFreedom or Resolute Onyx with 1-month dual antiplatelet therapy (DAPT) have the most supportive data.16
Patient with diabetes represent a challenging cohort. Most comparative trials of different DESs have shown no difference in effect of stent type between those with and without diabetes. In PLATINUM PLUS, there was no difference the in risk of the primary endpoint between those stented with PROMUS versus XIENCE (3.5% versus 3.5%, RR 1.00, 95% CI 0.62–1.60). However, in the sub-group with diabetes, XIENCE was favoured (7.8% versus 3.0%, RR 2.50, 95% CI 1.16–5.38, interaction p=0.05). This relationship, however, was not seen in the 5-year follow-up data of the preceding PLATINUM trial with a similar design.25,52 The comparison of BP DESs versus PP DESs in patients with diabetes was recently examined by Bavishi et al., who included 5,190 patients from 11 RCTs in a meta-analysis, focusing on current-generation stents.53 After a mean follow-up of 2.7 years, there were no differences in a range of outcomes, including target lesion revascularisation (RR 1.02, 95% CI 0.85–1.24, p=0.80) and stent thrombosis (1.66% versus 1.83%, RR 0.84, 95% CI 0.54–1.31, p=0.45) between the two stent types. There was no difference in this relationship between those patients with diabetes treated with and without insulin.
Stent for specific lesion types
Certain stents may perform better or at least may have been studied extensively for treating some complex lesions including left main stem, bifurcation lesions, chronic total occlusions, long lesions, and calcified lesions. For example, there have been many more dedicated trials with everolimus-eluting stents in unprotected left main disease or chronic total occlusions. Large left main stem with significant tapering after bifurcation may benefit from self-apposing Xposition-S. Ostial lesions would require DESs with good radial strength and better visibility. Calcified or tortuous lesions will benefit from good radial strength and better deliverability. DESs with thinner struts and fewer connectors between stent rings are easier to deliver. Operator preference, usually based upon individual experience and familiarity with a given platform for a specific anatomy and technique chosen, should be valued as much as evidence from trials in stent selection.
Is there any role for bare metal stents in current era?
DESs offered reduced in-stent restenosis rates when compared to BMSs, and this translates into clinical benefits on rates of revascularisation in particular. Use of DES in individuals with a high bleeding risk has traditionally been avoided due to the requirement for longer DAPT with DES. However, with current-generation DESs with reduced strut thickness and better/no polymers, this has become less of an issue. BioFreedom and Onyx can be used in patients with high bleeding risk. Studies have also supported use of DESs during primary PCI for ST-segment-elevation MI.54 Patients receiving second-generation DESs for the treatment of saphenous vein graft disease have also lower rates of major adverse cardiovascular events and mortality, compared with those receiving BMSs.55 Finally, the cost of DESs has also substantially reduced in recent years, removing BMSs almost completely from the contemporary practice.
On-going research and future directions
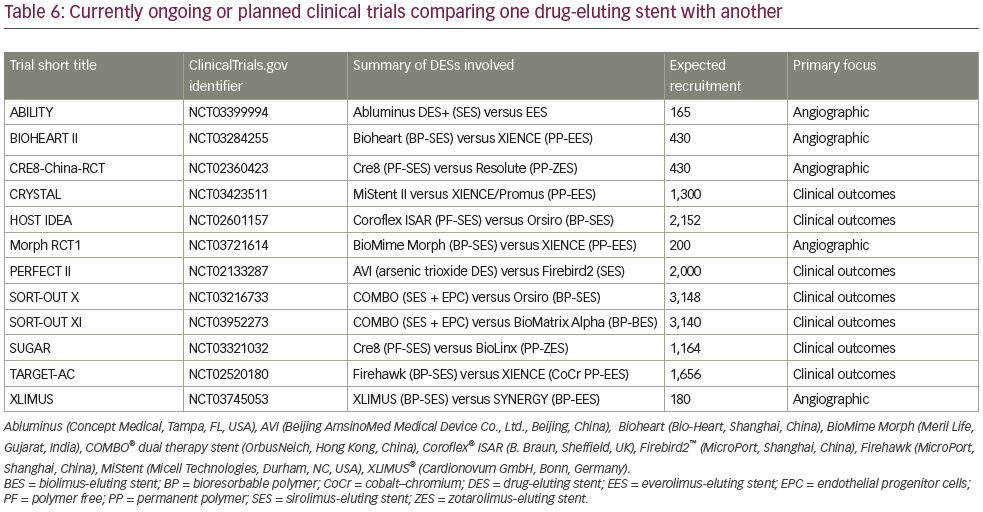
A number of RCTs are underway, or are planned, to compare the performance of DESs, whether these be of new or existing designs (Table 6). There does not seem to be any major trial of a new platform or alloy. However, new polymers and polymer-free stents are being tested in these trials. Some studies are also exploring novel anti-proliferative drugs, for example, arsenic trioxide rather than a ‘limus’ drug as an antiproliferative agent in the AVI® stent (Beijing AmsinoMed Medical Device Co., Ktd., Beijing, China) in the PERFECT II study. Further studies on stents coated with an anti-CD34 antibody to capture circulating endothelial progenitor cells, development of which has been ongoing for some years, are also being conducted.
Although beyond the scope of this review, drug-eluting bioresorbable vascular scaffolds also remain a prominent area of development within PCI technology. Constructed of biodegradable polymer, they are designed to maintain radial strength and deliver an antiproliferative drug after implantation but then to resorb altogether once all drug has been released. Benefits might include better long-term vessel dynamics and allowing subsequent bypass grafting at the site of PCI.56 Although outcomes, such as target lesion revascularisation, MI or death, appeared of similar incidence to DESs, rates of subacute stent thrombosis (1–30 days after implantation) were significantly greater, as was in-device late lumen loss.57 It remains to be seen whether technological development, for example constructing thinner strut bioresorbable vascular scaffolds, can enhance the risk–benefit profile of bioresorbable vascular scaffold to bring it back to clinical practice.
Many of these ongoing prospective studies also focus on angiographic outcomes or are non-inferiority of clinical outcomes but are not powered to detect superiority of one stent type over another. Unfortunately, it is unlikely that data comparing stent types head-to-head in RCTs powered for superiority analyses will become available in the foreseeable future.
Summary
The interventional cardiologist currently has a variety of stents available to treat obstructive coronary stenosis. DESs have been shown to be superior to BMSs in almost all patient and lesion types, and should be considered as default therapy in all PCI cases. A number of currently-available DESs have comparable safety and efficacy. DESs made of cobalt–chromium or platinum–chromium are generally preferable as they allow stents with thinner struts. It remains difficult to elucidate significant differences between the eluted ‘limus’ type drugs, and for clinical purposes, it seems likely that these drugs are of comparable safety and efficacy. Whilst BP DESs have theoretical advantages over PP DESs, at present these do not appear to be clinically significant in the short to medium term. This might be because current-generation PP DESs have thinner struts and exploit more biocompatible polymers than previous generations. However, the ultra-thin BP sirolimus-eluting stent, Orsiro, has shown favourable results in patients with stent thrombosis-segment-elevation MI. In patients with high bleeding risk, Onyx or BioFreedom DESs should be considered with 4 weeks DAPT. Clinicians should be aware of the properties of different stent types as well as supporting clinical data when making decisions regarding use of specific devices, and match these to the individual scenarios to provide best stent type for a particular patient and lesion.