Clinically relevant tricuspid regurgitation (TR) is a common disorder, affecting approximately 4% of people 75 years of age or older.1 If left untreated, severe TR results in volume overload and right ventricular remodelling. This eventually leads to symptomatic right-sided heart failure, along with increased morbidity and mortality. This disease is highly undertreated due to high complication rates and in-hospital mortality reaching up to 10% with surgical treatment, which until a few years ago was the only corrective therapy available.2 A novel and revolutionary approach has been developed for the treatment of TR in the form of transcatheter tricuspid valve intervention (TTVI) with different techniques. In this article, we aim to discuss in detail the edge-to-edge technique for the repair of significant TR with the TriClip device (Abbott, Santa Clara, CA, USA).
Aetiologies of tricuspid regurgitation
Depending on the aetiology, TR is further classified as primary or secondary.3 Primary TR occurs as a result of isolated valvular apparatus anomalies, which may be congenital or acquired. Whereas secondary TR, also known as functional regurgitation, is caused by extrinsic events affecting the tricuspid valve (TV) and represents the vast majority of the cases. The different aetiologies are summarized in Table 1.3
Table 1: Causes of tricuspid regurgitation
|
Primary tricuspid regurgitation |
Secondary tricuspid regurgitation |
|
1. Congenital:
2. Acquired:
|
|
ICD = implantable cardioverter defibrillator; RA = rheumatoid arthritis; SLE = systemic lupus erythematosus; SPAP = systolic pulmonary artery pressure.
Clinical presentation
There is no pathognomonic clinical presentation for patients with TR. When symptoms are present, they are usually related to right-sided heart failure.2 Furthermore, patients may present with symptoms of the underlying aetiology, such as fever in endocarditis. The severity of the symptoms does not correlate with the severity of the disease, as some patients with severe TR are asymptomatic.4
Anatomy and morphology
The TV has a complex anatomy and it consists of three leaflets (anterior, posterior and septal), the chordae tendineae, two papillary muscles, the tricuspid annulus, the right atrium and the right ventricle (RV).5 The anterior leaflet is the largest and most anatomically constant of the three; the posterior and septal leaflets are smaller and more variable in size and position.5
The number of TV papillary muscles varies. The larger anterior papillary muscle is most consistently present and supplies chordae to the anterior and posterior leaflets. A septal papillary muscle is absent in up to 20% of healthy people,6,7 and the septal leaflet may insert directly into the right ventricular free wall.
The normal tricuspid annulus is elliptical, longer in the septal-to-lateral dimension (from the aortic valve to the lateral free wall) than in the anterior-to-posterior dimension.8,9
Tricuspid annulus measurement is critical for decision-making regarding surgical and transcatheter interventions. It should be measured from its mid-septal to mid-anterior points in the apical four-chamber view in early diastole, when the annulus size is largest.10,11
The anterior papillary muscle provides chordae to the anterior and posterior leaflets, and the medial papillary muscle provides chordae to the posterior and septal leaflets. The septal wall gives chordae to the anterior and septal leaflets. In case of right ventricular dysfunction and dilation, these chordal attachments are the main mediators of TR, as they impair proper leaflet coaptation.5
Dilatation of the tricuspid annulus occurs mainly in its anterior/posterior aspect, because the small septal leaflet is fixed and there is little room for movement if the free wall of the RV were to dilate.12 This results in significant TR due to leaflet malcoaptation.13 Other factors involved in the variation of the severity of TR include right ventricular preload, afterload and right ventricular systolic function.14
2D transthoracic echocardiography is the most widely used technique to assess tricuspid annular size. However, when technically difficult or not feasible, cardiac computed tomography and cardiovascular magnetic resonance can also be used for planning of surgical and transcatheter procedures and may provide accurate annular measurements.15,16
Imaging of tricuspid valve
A clear understanding of leaflet morphology and pathophysiology leads to improved techniques for valve repair. The three TV leaflets can rarely be seen simultaneously on standard 2D transthoracic echocardiography and usually require scanning through different views. The recommended views for the assessment of the TV are: parasternal long-axis of the RV inflow, short-axis at the aortic valve level, apical four-chamber and subcostal windows.17 Transoesophageal echocardiography for the TV is sometimes used with the four-chamber view at 0 degree in the basal transoesophageal and oesogastric junction planes.18
The severity grading of the TR is based on valve morphology, leaflet coaptation, qualitative parameters (colour Doppler, pulsed wave Doppler and continuous wave Doppler), semi-quantitative (vena contracta) and quantitative (effective regurgitant orifice area [EROA] and regurgitant volume by the proximal isovelocity surface area [PISA] method) parameters. A vena contracta less than 3 mm suggests mild regurgitation, while measurements between 3 and 6.9 mm indicate a moderate TR. If the vena contracta width falls within the range of 7 to 13 mm, it signifies severe TR. A more extensive vena contracta, measuring between 14 and 20 mm, corresponds to a massive degree, and a width exceeding 20 mm is classified as torrential TR. In addition to vena contracta width, the severity of TR can be evaluated based on the EROA. An EROA of less than 20 mm² corresponds to mild TR, while measurements between 20 and 39 mm² indicate a moderate degree. If the EROA falls within the range of 40 to 59 mm², it suggests severe regurgitation. A more extensive EROA, ranging from 60 to 79 mm², is associated with massive TR, and an EROA exceeding 80 mm² is indicative of torrential regurgitation.18,19
The impact of TR is also evaluated by measuring right atrial and RV dimensions, the systolic function of the RV (Tricuspid Annular Plane Systolic Excursion [TAPSE], peak systolic velocity [pulsed wave (Pw) s’] in tissue Doppler, fractional area change, RV ejection fraction), pulmonary pressures, inferior vena cava diameter and the suprahepatic veins (systolic flow reversal is suggestive of severe TR). The parameters associated with RV dysfunction are: fractional area change <35%, TAPSE <17 mm, Pw s’ <9.5 cm/s and longitudinal strain <20%, ejection fraction <45%. A tricuspid annular diameter of ≥40 mm or 21 mm/m2 in end diastole in four-chamber view is suggestive of severe annular dilatation.20
TR is graded as mild, moderate and severe. Severe TR can be further subdivided into massive and torrential subcategories that better represent prognosis.21–24
The following are also additional criteria for TR severity: a TR continuous wave Doppler jet that is dense and triangular in shape with early peaking, PISA radius >0.9 cm for a Nyquist limit of 28 cm/s, and a regurgitant volume ≥45 mL. Furthermore, dilated right-side cavities are suggestive of the chronicity of the TR.22–24
Patient selection and risk stratification
Patient selection and evaluation by a multidisciplinary heart team is a vital step prior to any potential intervention to individualize decision-making and opt for the best therapeutic approach. 2D/3D transthoracic and transoesophageal echocardiography is the test of choice for diagnosing, grading and defining the mechanism of TR.2 Patient evaluation must be performed after ensuring that they are already on optimal medical therapy. Furthermore, it is of utmost importance to achieve adequate diuresis and ensure euvolaemia prior to evaluation in order to avoid a false worsening of TR severity, which would lead to erroneous patient stratification. Additional advanced multimodality imaging may be helpful when echocardiography is either of poor quality or inconclusive. Right and left heart catheterization should be performed. Right heart catheterization is done to assess the severity and mechanism of pulmonary hypertension (PHT), measure pulmonary vascular resistance, RA pressure/pulmonary capillary wedge pressure ratio, pulmonary artery pulsatility index and the reversibility of PHT. Left heart catheterization is done to rule out coronary artery disease and specifically evaluate the right coronary artery (RCA) status and course.1 A blood work is performed including, but not limited to, complete blood count, renal and hepatic function, inflammatory status, volume overload and malnutrition. The use of TRI-SCORE can help further stratify the patients. TRI-SCORE is a recently developed score consisting of clinical, echocardiographic and blood test parameters for the prediction of post-operative in-hospital mortality following isolated TV surgery, which can be calculated online.25 Thus, a high TRI-SCORE favours a transcutaneous approach, rather than a surgical one.26
Classically, surgery has been considered the first-line of treatment for severe TR. However, if after a global evaluation the patient is deemed inoperable or at a high surgical risk, evidence suggests that TTVI is associated with a lower rehospitalization and all-cause mortality in comparison with optimal medical therapy alone.27 Selecting the optimal device to perform the TTVI can be challenging due to the presence of different devices and techniques on the market with no head-to-head clinical trials to compare outcomes. Therefore, careful study of the anatomy and physiology is warranted to best select the treatment modality. The devices are classified according to the mechanism of repair and are as such grouped under four major categories: annuloplasty devices, replacement devices, caval valve implantation and coaptation devices (edge-to-edge repair).2 TriClip, a coaptation device, which is currently the most widely used approach to treat severe TR, approximates the tricuspid leaflet edges and embodies the so called edge-to-edge repair. A proposed algorithm to guide device selection based on the mechanism and characteristics of the regurgitant lesions is provided in Figure 1. Furthermore, Table 2 provides on overview of optimal conditions and complicating factors for the TriClip procedure.2
Figure 1: Proposed algorithm to guide transcatheter tricuspid valve intervention device selection
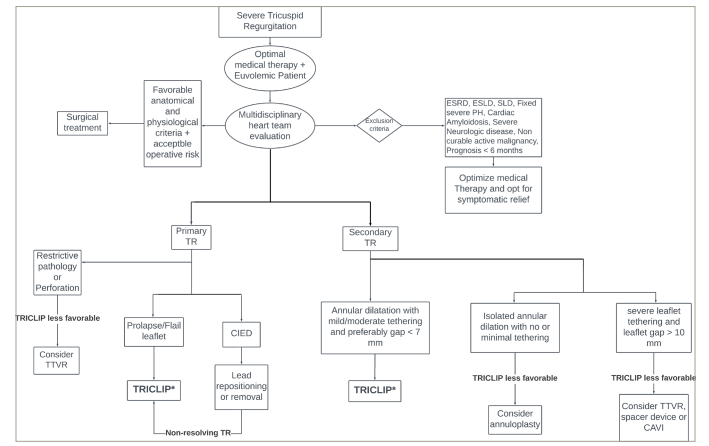
CAVI = caval valve implantation; CIED = cardiovascular implantable electronic device; ESLD = end-stage liver disease; ESRD = end-stage renal disease; PH = pulmonary hypertension; SLD = severe lung disease; TEER = transcatheter edge-to-edge repair; TR = tricuspid regurgitation; TTVR = transcatheter tricuspid valve replacement.
*Refer to Table 2 for ideal and complicating factors of technique.
Table 2: Ideal and complicating factors for TriClip intervention
|
Approach |
Optimal conditions |
Complicating factors |
|
TriClip (edge-to-edge repair) |
• Coaptation defect <7 mm • Adequate leaflet mobility • Anteroseptal jet location • Confined leaflet prolapse or flail leaflet • RV lead with no primary leaflet obstruction |
• Gap size >8.5 mm • Thick and/or short leaflets • Perforated leaflet • Difficult echocardiographic visualization • CIED RV lead leaflet impingement • Anteroposterior jet location • Severe leaflet tethering |
CIED = cardiovascular implantable electronic device; RV = right ventricle.
TriClip interventions procedural steps
The TriClip System is an edge-to-edge repair technique with a design that has been extrapolated from the MitraClip device (Abbott, Santa Clara, CA, USA). Originally, the technique was used in the treatment of mitral regurgitation, resulting in experience accumulation in the field of transcatheter interventions.28 Currently, the TriClip G4 device (Figure 2) has four different implant sizes that can be used. The TriClip apparatus consists of two main components: the clip delivery system (CDS) and the steerable guide catheter (SGC).29 The CDS includes an implantable clip with grippers, a delivery catheter and a steerable sleeve, used to guide and advance the clip. The SGC consists of a dilator, control knobs (actuator, F/E, S/L and ± ), levers and fasteners used to steer and actuate the guide and the CDS. The F/E knob flexes and extends the delivery catheter. The S/L knob, introduced with the G4 model, enables septal and lateral motion. The ± knob straightens and curves the guide, thus adjusting the height of the system relative to the valve. Furthermore, withdrawing the device orients it posteriorly, advancing it orients it towards the aorta; clockwise rotation orients it towards the septum and anticlockwise turns it laterally. Two techniques have been described to reduce TR: the triple orifice technique, which consists of placing the clips in a central position between the septal leaflet, anterior leaflet and posterior leaflet. The second and more commonly used approach is the bicuspidation technique, in which the clips are most often placed between the septal and anterior tricuspid leaflets.
Figure 2: TriClip G4 transcatheter edge-to-edge repair system
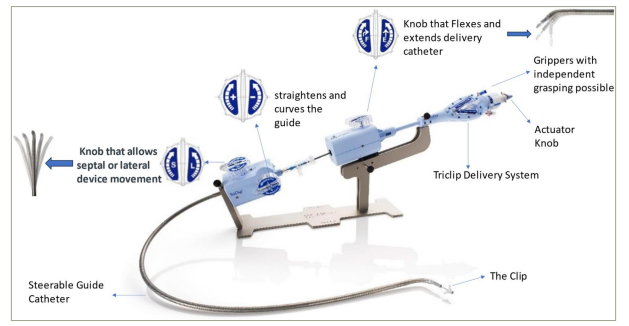
Reprinted with the permission of Abbott Medical France, Paris, France.
The procedure starts by performing general anaesthesia on the patient. It is generally done under transesophageal echocardiography (TEE) and fluoroscopy guidance.29 Right femoral venous access is obtained and a 24F sheath is inserted. Then, preclosure is performed with two Perclose ProGlides (Abbott Vascular, Santa Clara, CA, USA). Unfractionated heparin is administered to achieve a goal activated clotting time of 250–350 seconds throughout the procedure.30 A stiff wire is advanced to the superior vena cava. Then, the SGC is exchanged with the sheath and advanced over the wire, with the ± knob turned to 180 degrees towards the “-” sign to help straighten the tip and facilitate advancement.30 Occasionally, straddling the superior vena cava (SVC) might be needed. The device is then unsheathed in the right atrium and the ± knob returned to the neutral position. The wire is retracted into the device to have a parallel orientation of the device with the septum. Then, the F/E knob is flexed to steer the tip of the device towards the TV and the S/L knob is turned towards “L” to free the device from the septum. At this point, the device should be in the right atrium just above the TV. The device is the opened to examine the functionality of the grippers. Once adequate functionality is ensured, the device is closed and advanced to the RV under TEE and fluoroscopic guidance. At this stage, TEE guidance with transgastric short axis view, the right ventricular outflow tract (RVOT) outflow view and the four-chamber view are used interchangeably along with left anterior oblique (LAO) projection on fluoroscopy to optimize and fine tune the device location and coaxiality with the TV leaflets in preparation for grasping them.30 The transgastric view is the most helpful in locating the maximal regurgitation site and measuring the gap size and so helps determine the best clipping location. In most cases, the anterior and septal leaflets are clipped. So, the device is now opened and the operator performs fine manipulations to ensure maximum overlap of TV leaflets and clip arms. With the old system, the leaflets are grasped simultaneously; however, with the G4 TriClip model it is possible to perform independent grasping with separate activation of the grippers, which further allows optimal leaflet grasping. After ensuring proper device positioning and orientation on TEE and fluoroscopy a slight pull back is carried out and leaflet grasping is performed. Then, procedural success is evaluated by fluoroscopy and TEE. It is defined by the reduction of regurgitation by one or more grades without creating a residual valve stenosis and the absence of partial leaflet detachment or device margination. Once the procedure is deemed successful by the operating team, the clip is deployed and the system is retracted. If a residual significant TR is still present after clip deployment, more than one TriClip may be placed until an optimal result is obtained. Femoral venous access closure is completed with the Perclose ProGlide sutures placed at the beginning of the procedure.2
A video with a step-by-step description of the procedural steps and techniques of TriClip implantation is available online.31
Safety and outcomes
The recently published randomized controlled trial, TRILUMINATE pivotal trial (ClinicalTrials.gov identifier: NCT03904147), included patients with severe TR at intermediate or high risk of mortality with TV surgery.32 In this trial, transcatheter edge-to-edge repair with TriClip reduced TR to moderate or less severity in 87% of patients (Figure 3) and demonstrated an excellent safety profile. This reduction in regurgitation severity was associated with improvements in quality of life. However, it failed to reduce the incidence of death or tricuspid-valve surgery and the rate of hospitalization for heart failure at 1 year.32 Furthermore, the bRIGHT study (ClinicalTrials.gov identifier: NCT04483089), a prospective, single-arm, open-label, multicentre, postmarket registry, demonstrated that transcatheter tricuspid edge-to-edge repair of significant TR via TriClip was able to significantly improve functional capacity and quality of life at 30 days.33As a result TTVI, for the first time ever, was mentioned in the 2021 European Society of Cardiology/European Association for Cardio-Thoracic Surgery Guidelines for the management of valvular heart disease as a therapeutic potion to treat, inoperable, anatomically eligible patients with significant TR.34
It was demonstrated that TTVI is a safe and effective option for the treatment of symptomatic or severe TR in patients not suitable for surgery.32
Figure 3: Tricuspid regurgitation before and after TriClip procedure
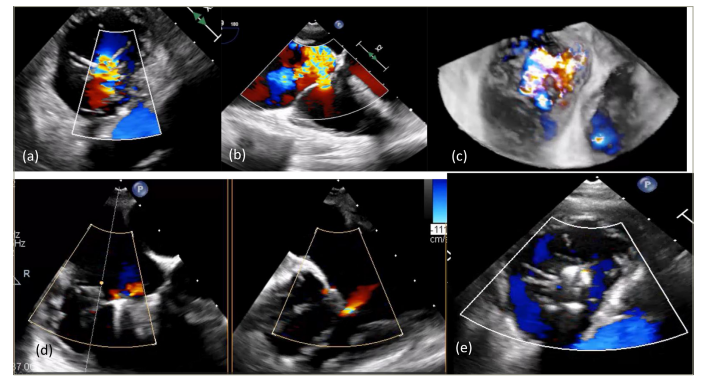
a–c: Different views of severe tricuspid regurgitation seen on intraprocedural transesophageal echocardiography (TEE) evaluation before TriClip placement.
a: transgastric view; b: mid-oesophageal view; c: 3D transgastric view.
d and e: Significant reduction in tricuspid regurgitation (TR) and near complete resolution of TR seen on the intraprocedural TEE assessment after TriClip procedure.
d: biplanar mid-oesophageal view; e: transgastric view.
Images from patients at the Institut Mutualise Monstsouris, Paris, France.
Postprocedural antithrombotic treatment
TTVIs represent an increased risk of thrombosis due to the low pressure circulation of the right side of the heart. The optimal antithrombotic therapy following TTVI remains controversial.35 Approximately 90% of patients undergoing TTVIs have atrial fibrillation as a comorbid condition and, as such, anticoagulants are reintroduced after the procedure and continued lifelong.35 Patients with no indication for systemic anticoagulation may benefit from antiplatelet therapy consisting of 4 weeks of aspirin plus clopidogrel, followed by aspirin daily for life.36
Conclusion
Significant TR is associated with high patient mortality and morbidity. This entity has long been undertreated due to a high complication rate with surgical repair. Transcatheter edge-to-edge repair with TriClip is a safe alternative to surgical treatment and was proven to reduce TR severity and significantly improve quality of life.