Background: The feasibility and safety of same day discharge for transvenous ICD systems is well established. Subcutaneous implantable cardioverter defibrillators (SQ-ICD) are increasingly being implanted as an alternative to transvenous ICDs. The feasibility as well as time and cost savings associated with same day discharge after implantation of these devices needs to be investigated.
Purpose: To evaluate the safety, time savings, and cost reduction associated with same day discharge of primary prevention SQ-ICD implants.
Methods: We prospectively analysed 24 consecutive patients who underwent SQ-ICD implantation for a primary prevention indication. Patients were excluded if: 1) they were undergoing lead or device revision; 2) lived greater than 30 miles from an emergency department; 3) did not have a driver or responsible party to stay with them overnight; 4) developed a post-procedure complication while in the hospital; or 5) the procedure was completed after 1 pm.
A pilot protocol assigned specific roles for the implanting physician, nurse practitioner, EP lab staff, and device clinic staff to ensure a smooth same day discharge and coordination for follow up. The patient received a next day phone call from the nurse practitioner and was scheduled for an incision check in 1 week in the device clinic.
Results: Of the 24 patients, 54% were successfully discharged on the same day. At the 1-week incision check, there were no site infections. Two patients were readmitted, one in each group, within 30 days of discharge (one for CHF exacerbation, one for symptoms of fatigue).
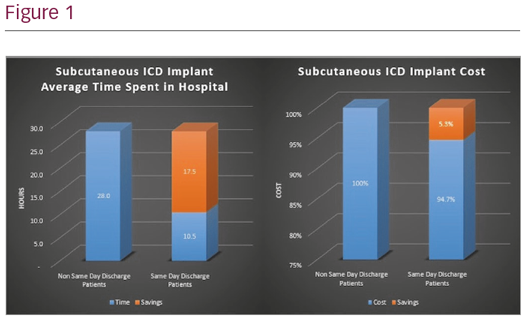
Analysis of average time spent in the hospital per patient demonstrated a time savings of 63% with same day discharge. This improved hospital bed access by 17.5 hours per patient.
Variable cost, calculated by applying hospital cost to charge ratios, demonstrated a 5.3% reduction in cost of care per patient when discharged the same day. This savings was driven by a reduction in nursing cost. This analysis does not include non-billable costs accrued as a consequence of the outpatient current procedural terminology classification for ICD implant, which does not allow billing the overnight resource utilisation.
Conclusion: In a small cohort of patients undergoing SQ-ICD implantation, same day discharge is safe and feasible. Procedure-related complications were not apparent and the reduction in healthcare costs incurred has immense benefit in today’s healthcare environment.