Background: Cardiac contractility modulation (CCM) is a new device based technology which applies non-excitatory electrical stimuli during the absolute refractory period, which enhances the strength of cardiac contractions. Increasing evidence exists suggesting that CCM improves symptoms in heart failure if various selection criteria are fulfilled. It is unknown how many people might benefit from this therapy. The aim of this study is to analyse an unselected sample of heart failure patients requiring hospital admission to establish what percentage of patients would meet the current criteria for CCM therapy.
Methods: Over one calendar year (2018) all patients admitted to two district general hospitals (Eastbourne District General Hospital and Conquest Hospital, Hastings) in the UK who were classified with a diagnosis of heart failure, were audited for eligibility for CCM therapy. The selection criteria were 1) EF 25-45%, 2) QRS duration less than 130 ms, 3) NYHA class 3 or 4 and 4) treated for heart failure for at least 90 days and on stable medications. Exclusion criteria included 1) significant valvular disease, 2) permanent or persistent atrial fibrillation, 3) biventricular pacing system implanted or QRS duration more than 130 ms and 4) patients not suitable for device therapy due to palliative treatment intent.
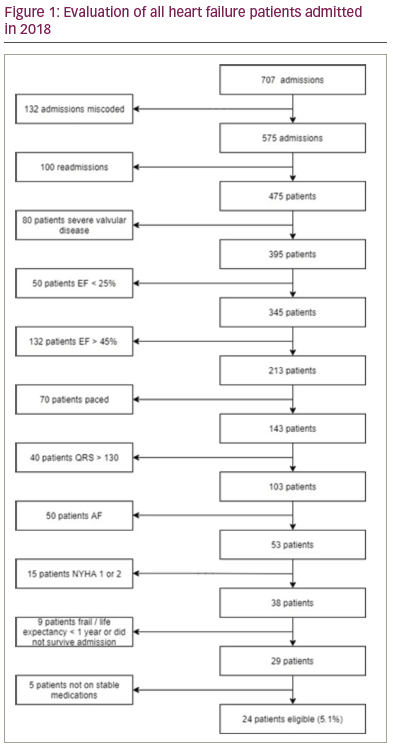
Results: 475 patients were admitted with heart failure during the study period. From this group 24 (5.1%) patients fulfilled the criteria for CCM therapy (figure 1). The mean age of patients was 70.8 ± 10.2 and the mean ejection fraction was 32.5 ± 7.4%. The majority of patients were male (70.8%) and the majority (75%) had ischaemic cardiomyopathy as the cause of their heart failure. There were no significant differences in age, QRS duration, gender, aetiology of heart failure, diabetes, COPD or CKD between patients with severe left ventricular (LV) dysfunction versus those with moderate LV dysfunction. Patients with severe LV dysfunction were significantly more likely to be hypertensive (10 (62.5%) vs
1 (12.5%), p=0.03).
Conclusion: Only 5.2% of all patients presenting with heart failure might benefit from cardiac contractility modulation therapy. This is a smaller proportion of the overall heart failure population than previously estimated. However, this population has no other current option for device therapy of their condition. This may have cost implications to the health service and may encourage the uptake of this novel therapy.