Several smart, connected and direct-to-consumer wearable devices capable of detecting cardiac arrhythmias have proliferated in the marketplace in the last decade. Increasingly, these devices are being marketed as “medical grade” in addition to “wellness” devices.1 New iterations of smartwatch and smartphone technology incorporate biosensors and advanced artificial intelligence (AI) algorithms equipped to diagnose cardiac arrhythmias; examples include the Apple Watch 7 (Apple Inc, Cupertino, CA, USA) and Samsung Galaxy Watch 4 (Samsung Group, Seoul, Republic of Korea). Wearable technologies have been touted as a paradigm shift in healthcare in the medical literature because they offer the opportunity for early diagnosis in asymptomatic consumers and, as AI develops, possibly negate the need for clinical reasoning to suspect the presence of certain cardiac conditions.2 This marks a significant deviation from the usual patient–physician relationship, where the physician orders investigations based on clinical reasoning to diagnose a condition.3
Remote monitoring devices for detecting cardiac arrhythmias, such as Holter monitors and implantable loop recorders, are in widespread use; however, these devices are often cumbersome to carry around or require an invasive implant procedure.4 Newer single-lead electrocardiogram (ECG) monitors, such as the ZioPatch (iRhythm Technologies, San Francisco, CA, USA), and compact personal ECG devices, such as Kardia Mobile (AliveCor, Mountainview, CA, USA), have increasingly been adopted by electrophysiologists to detect paroxysmal rhythms. However, these devices are beyond the scope of this review. Articles within the published literature were screened using the search terms “remote monitoring”, “wearable technology”, “smartwatches” and “cardiac arrhythmias”.
In this article, we focus on innovative and potentially more cost-effective wearable devices that use biosensors embedded within smartwatches, smartphones, necklaces, rings and textile garments. We evaluate the current landscape, discuss currently available devices and associated clinical trials, and discuss potential advantages and difficulties of integrating consumer devices into the clinician’s workflow. Whether wearable technology can accurately and reliably facilitate the passive diagnosis of potentially fatal cardiac arrhythmias remains an exciting prospect in cardiovascular medicine.
Arrhythmias
Abnormal cardiac rhythms, including brady- and tachyarrhythmias, carry a significant health burden, often due to their paroxysmal nature and low detection yield, which can lead to delayed diagnoses.5 Atrial fibrillation (AF) is the most common cardiac arrhythmia worldwide. It is associated with reduced quality of life, risk of significant morbidities, such as heart failure, and a five-times increased risk of stroke and significant mortality.6 Globally, 37.5 million people have AF. This amounts to 0.51% of the global population, with its prevalence increasing by 33% over the last two decades, and its prevalence is estimated to double in Europe by 2060, which will confer a significant economic and health burden.6,7 A recent economic analysis conducted within the EU estimated that the annual cost of illness resulting from AF per patient ranges between €5,586 and €7,341 per year.8 As the global population ages and the availability of therapeutic innovations to extend life increases, we expect the overall cost of managing comorbidities to rise.
A validated and clinically proven methodology for detecting subclinical AF could significantly reduce secondary complications and comorbidities associated with this disease and, thus, reduce the societal burden and costs to quality of life imposed by this illness. To date, the gold standard for diagnosing AF remains the 12-lead ECG, although the recent European Society of Cardiology 2020 Guidelines for Management of AF have recognized and included single-lead ECG as acceptable for diagnosis.6 Over the last decade, remote monitoring technologies have been developed to extend the monitoring timeframe and improve the detection yield of clinically significant arrhythmias. Further, advances pioneered by consumer technology giants such as Apple (Apple Inc, Cupertino, CA, USA) have attempted to assist with the diagnosis of clinically significant episodes of AF over years rather than days using existing non-invasive ambulatory monitors.9 As billions of dollars continue to be invested into further advancing biosensors for detecting AF, a vision whereby the global population are screened through mobile health (mHealth) technology with low rates of false positives continues to be investigated, with an increasing number of clinical trials assessing the effectiveness of device-based arrhythmia detection.
Wearable technologies: Is adoption increasing?
mHealth devices and, specifically, wearable technologies have been proposed as a new-age solution for replacing invasive, less cost-effective and more labour-intensive methods for monitoring arrhythmia. With the development of increasingly accurate, real-time measurements of cardiovascular biometrics, the general population has gained access to a multitude of devices with in-built AI algorithms to provide diagnostic information.10 Numerous wearable devices, such as the Fitbit (Fitbit Inc, San Francisco, CA, USA) models and the Apple and Samsung (Samsung Group, Seoul, Republic of Korea) watches, have recently been made available to the health-conscious consumer, leading to an explosion of wearable devices being sold. It is estimated that, in 2022, 20% of the population of the USA will own a wearable device that will help consumers actively engage in their overall health and fitness level.11 Furthermore, 75% of consumers above the age of 65 view wearable technology as a positive development in digital healthcare.12 At present, the Apple Watch is the most popular among consumers; other companies, including Samsung and Fitbit, have also developed wearable technologies to compete with Apple, who held the largest market share in 2021.13 As the adoption of wearable technology increases, so do the opportunities to further hone AI systems with more data points. In theory, more data points should improve the ability of devices to accurately detect and predict arrhythmic abnormalities. A recent high-impact research report valued the global wearable technology market at approximately US \$25 billion, with growth projected at a compound annual growth rate of 22.9%, whereby conservative estimates put market penetration at US \$140.1 billion by 2027.14
How do wearable devices detect arrhythmias?
In the field of electrophysiology, wearable devices use either photoplethysmography (PPG) or electrode-based ECG technology integrated within the hardware to delineate cardiac rhythms.15 PPG technology has been postulated as a low-cost, non-invasive solution for continuously monitoring the cardiac cycle to detect possible arrhythmias. It uses an optical technique to derive a waveform by measuring the pulsatile changes in microvascular blood volume that correspond with the cardiac cycle.16 The peak interval between each pulsation has been identified to correlate with the cardiac R–R interval.17 AI algorithms developed using deep neural network machine-learning techniques are programmed into wearable devices to distinguish between AF and sinus rhythm (SR).18 In addition, newer models of smartphones and smartwatches offer additional built-in accelerometer data in order to filter out motion artefacts to improve signal quality.19
The 12-lead ECG remains the backbone of arrhythmia diagnosis; however, single-lead ECG technology can be incorporated into compact wearable devices. In this proposed model, PPG-identified arrhythmias signal the device to prompt users to perform a single-lead ECG through the same device to confirm an abnormal rhythm.
AF remains the primary arrhythmia of interest in the development of this technology, with evidence supporting precise and continuous AF detection by many common wearable technologies, thus offering health systems an excellent opportunity to screen for AF on a large scale in a population at risk of stroke.20
The current landscape: Where are we now?
Extensive research, funding and technological advances have been allocated to evaluate the diagnostic capabilities of PPG- and ECG-integrated wearable devices. A search on ClinicalTrials.gov with search terms associated with digital and remote monitoring reveals over 1,000 trials that are recruiting or have been completed.11 The multi-billion-dollar global technology giants have been leading the way in conducting uncontrolled yet large-scale prospective trials to assess the accuracy of wearable devices. Within 9 months, the Apple Heart Study (Apple Heart Study: Assessment of wristwatch-based photoplethysmography to identify cardiac arrhythmias; ClinicalTrials.gov identifier: NCT03335800) remotely recruited 419,297 patients who e-consented to have their heart rate continuously monitored using PPG technology via an Apple Watch.21 The proprietary algorithm informed only 0.52% of all study participants of a possible arrhythmia; a telehealth visit was arranged to investigate this finding further, and a single-lead ECG patch was sent to these participants. Only 20.8% returned the ECG patches, 34% of whom screened positive for AF, thus revealing a weak study diagnostic yield of 0.04% and a modest positive predictive value of 0.84.9
A similar study (Huawei Heart Study: Mobile photoplethysmographic technology to detect atrial fibrillation; Chinese Clinical Trial Registry identifier: ChiCTR-OOC-17014138) conducted by Huawei (Huawei Technologies Co Ltd, Shenzhen, China) yielded similarly modest results.22 Of the 187,912 patients they recruited, 0.23% received notifications for irregular rhythm; their study followed up 67% of those who received notifications and obtained a diagnosis yield of 0.12%.23 Furthermore, Fitbit, recently acquired by Google (Google LLC, Mountain View, CA, USA), have recently released a smartwatch with ECG integration and recruited 455,699 participants to provide critical insights into passive arrhythmia monitoring.24,25
Both the Apple and Huawei studies highlight the role of the evolution of wearable technologies within cardiovascular monitoring; however, both studies included participants of a higher socio-economic background with a mean age of under 40 for both studies, so its use in optimizing cardiovascular health in populations with a greater prevalence of AF and limited technological literacy is yet to be determined.
Numerous on-going studies are investigating the feasibility of adopting wearable technologies for people aged 65 or over, who are at greatest risk of arrhythmia and its complications. The SAFER Wearables study (A study of the acceptability and performance of wearables for atrial fibrillation screening in older adults; ClinicalTrials.gov identifier: NCT04715555) aims to assess the subjective experiences of older patients (65 years or older) using the Zenicor-ECG (Zenicor Medical Systems AB, Stockholm, Sweden), in addition to device performance.26 When released, its results will likely be an asset to the on-going exploration of the feasibility and usefulness of long-term adoption of wearable technology diagnostic models for those at greatest risk of arrhythmia and its associated cardiac complications. A clinical trial being conducted by the authors, REMOTE-AF (Remote monitoring of AF recurrence using mHealth technology; ClinicalTrials.gov identifier: NCT05037136), aims to provide data on the use of wearable technology for monitoring arrhythmia recurrence after an invasive ablation procedure in individuals with long-standing persistent AF to assess whether this technology can be relied on as a diagnostic tool.27
A large meta-analysis evaluating the diagnostic accuracy of 10 studies investigating PPG signals collected from smartphones and smartwatches (via finger and facial skin) and analysed by machine learning algorithms has concluded that mass screening for arrhythmias, particularly AF, using smartphones and smartwatches remains a viable and reliable proposition.28 Nonetheless, the low detection yield of arrhythmias and unknown clinical outcomes are important to note and highlight the need for further research. The HEARTLINE study (HEARTLINE – A heart health study using digital technology to investigate if early AF diagnosis reduces the risk of thromboembolic events like stroke in the real-world environment; ClinicalTrials.gov identifier: NCT04276441) has been recognized as the first study in those aged 65 years or over to assess whether app-based devices that detect AF can reduce the risk of stroke and cardiovascular mortality.29 Lastly, the ARTESiA trial (Apixaban for the reduction of thrombo-embolism in patients with device-detected sub-clinical atrial fibrillation; ClinicalTrials.gov identifier: NCT01938248) will attempt to assess stroke and embolic event outcomes during a 10-year follow-up period in patients treated with either apixaban or aspirin following the identification of device-detected subclinical AF.30 Trial data from both these prospective clinical trials could lead to changes in guideline recommendations.
In addition to smartwatches and smartphones, PPG biosensors have been integrated into other wearable technologies, such as necklaces (ECG Necklace, Maastricht Instruments BV, Maastricht, Netherlands), rings and textile garments.15 Moreover, the US Food and Drug Administration and the EU, via a class IIa medical device certification, have authorized a medical-grade smart T-shirt (KeeSense™, Chronolife SAS, Paris, France), which can perform a 3- to 12-lead ECG with automated AI interpretation through textile nanoelectrodes.31
PPG sensors embedded within jewellery at different body sites have yielded promising results, with finger PPG producing the highest quality analysable waveform.32 CART (SkyLabs Inc, Seongnam, Republic of Korea), a ring-type wearable device, was evaluated in a prospective study, which yielded promising results in a cohort of patients who underwent direct-current cardioversion for persistent AF.33 PPG signals from the finger were transferred wirelessly to a smartphone application and analysed using a deep learning algorithm. The study observed a sensitivity for AF detection of 99.0%, a specificity of 94.3%, a positive predictive value of 95.6% and a negative predictive value of 98.7%. A novel single-lead ECG device embedded in a necklace was found to produce comparative results, detecting both AF and SR with a sensitivity and specificity greater than 95.0%.34
The current landscape shows the potential to advance the clinical usefulness of wearable technologies. With continued research focused on measuring clinical outcomes to reduce stroke risk and cardiovascular mortality, wider clinician adoption is likely to follow if outcome data can be critically appraised alongside a balanced evaluation of the advantages and disadvantages to patients.
Limitations
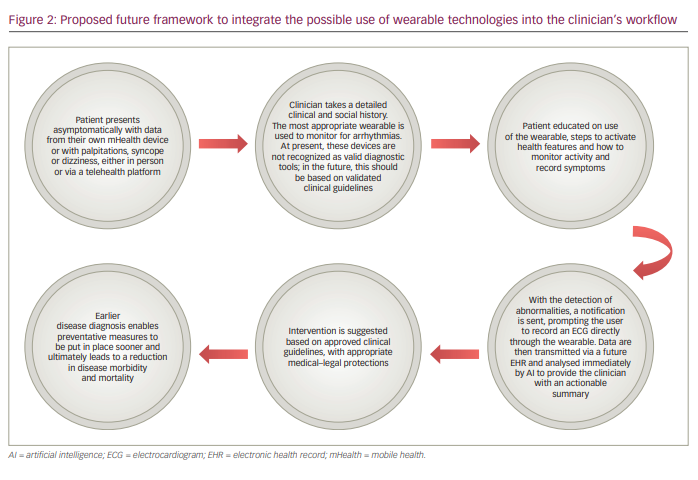
Figure 1 shows the potential advantages and challenges of wearable technologies. In turn, in Figure 2 we propose a framework for the integration of data from wearable technology into the clinical cardiology workflow to both monitor for rhythm abnormalities and to support lifestyle advice, with the aim of improving cardiovascular health. At present, these devices are not recognized as a means for detecting arrhythmias. Our framework is a glimpse into a future where these technologies are universally accepted and recognized as valid adjunctive tools in the armamentarium for diagnosing arrhythmias.
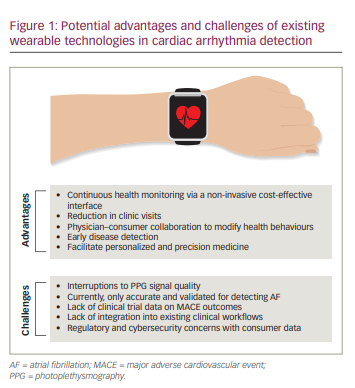
The primary benefit of wearable technology in the clinical cardiology workflow is to detect arrhythmias, as demonstrated in the non-invasive, continuous, passive monitoring of heart rate.35 In addition, as devices become more compact, lightweight and easier to operate, greater adoption among the population is likely to ensue.36 These changes should empower consumers to engage positively with their health, thus supporting the development of a collaborative relationship with their treating physicians, encouraging early presentation of symptoms to healthcare practitioners and reducing the economic and health costs caused by late diagnosis and secondary comorbidities.
The considerable potential of wearable technologies can be harnessed to widen the access to cardiac diagnosis in underserved world populations, in which the secondary consequences of poverty and inadequate access to healthcare is understood to adversely affect long-term outcomes in cardiac disease.37 Increased access to wireless connectivity, more sophisticated AI algorithms and the ongoing reduction in device cost will lead to a ubiquitous penetration of trends in ownership, giving many more people access to the potential diagnosis of arrhythmia and telehealth follow-up. Nevertheless, the widespread acceptance of wearable technologies as gold-standard diagnostic technology or management aids will require the limitations of this novel technology to be overcome.
PPG signal quality has been known to be significantly affected in a number of real-world scenarios. Background noise, variations in skin temperature, skin pigmentation and ambulation can interfere with signal quality, resulting in the loss of valuable data.38 The WATCH AF study (Smartwatches for detection of atrial fibrillation [WATCHAF]; ClinicalTrials.gov identifier: NCT02956343) recruited 672 participants with a commercially available AF-sensing wrist-worn device; they registered 21.8% of the PPG readings as uninterpretable.39 Device inaccuracy in distinguishing rhythms in states of extreme heart rates is a further area of concern, and if real-world use reproduced such a high percentage of unclassifiable readings as in WATCH AF, clinical acceptance and usefulness would be limited.40
Regulatory and logistical burdens also exist when implementing a framework for the widespread adoption of wearable devices. Concerns around data security and privacy, lack of integration of automated data summaries into existing clinical workflows and decreasing adherence after 3 months of use need to be addressed before wearable technologies are realistically incorporated into clinical pathways.28 Lastly, low but significant false-positive rates may lead to unnecessary investigations, which fuel patient anxieties and may expose participants to the adverse effects of unnecessary investigation or treatment. As large consumer technology companies, healthcare providers and healthcare practitioners continue to work in partnership to enhance the user experience and validity of data outputs of wearable remote monitoring devices, the benefits of these technologies may soon outweigh their inadequacies. Our proposed framework (Figure 2) attempts to propose a significant change to existing practices by incorporating wearable technologies into the cardiologist’s or general practitioner’s diagnostic and therapeutic armoury.
Where are we headed?
In August 2020, the Heart Rhythm Society, in conjunction with the European Heart Rhythm Association, described the progress made in mHealth technologies as an important frontier in cardiovascular health evaluation, while acknowledging the diagnostic limitations of existing devices.41 As clinical trials attempt to provide data on clinical validity, health outcomes and cost-effectiveness, greater adoption of wearable technology may follow.
Use cases for wearable technology in the field of arrhythmia include the following:
- widespread screening of the general population
- continuous monitoring of patients at high risk of developing cardiac arrhythmias
- monitoring for recurrence of AF in patients with AF
- managing rate-control therapies in patients with permanent AF
- targeted anticoagulation or anti-arrhythmic treatment following an evaluation of AF burden
- assessing patients prior to presenting for rhythm-control strategies, such as direct-current cardioversion, to minimize unnecessary hospital visits.42–44
Amid the global coronavirus disease 2019 (COVID-19) pandemic, and with expectations of future pandemics, remote technologies may prove invaluable in providing uninterrupted care to patients with cardiovascular disease.45
With the continual development of AI algorithms and quantum computing, which enables complex computational problems to be solved faster and with more accuracy, a single connected device containing sensors and software with the ability to process PPG waveforms, data analysis and ECG confirmation to suggest a validated intervention may revolutionize the management of cardiac arrhythmias.11 As these devices spread through existing healthcare infrastructures, regulatory policies and clinical guidelines will need to be developed after careful evaluation of the obtained data to provide high-quality recommendations on use. Moreover, concerns surrounding liability for arrhythmia notifications, patient privacy, adherence to General Data Protection Regulation laws and the risk of overburdening health systems with workloads generated by early and asymptomatic detection will need to be addressed.46 In addition, the economic impact of consumer-led arrhythmia detection through wearable technologies remains relatively unstudied.40 As an increased volume of clinical events is detected in clinically well patients, further work is needed to assess whether there are increases in unnecessary downstream testing, which may lead to unintended health or economic consequences, as this model differs significantly from current practice whereby physicians order specific investigations based on history, examination and clinical acumen.
Education of healthcare professionals to improve awareness of the potential usefulness of mHealth will be required to facilitate the translation of its use into daily practice. As adoption grows and development expands into arrhythmias other than AF, collecting vast swathes of digital health data, in combination with in-built sensing of additional physiological parameters, may allow individual cardiac phenotyping and enable the growing discipline of personalized medicine to flourish.28,47,48
Conclusion
Accelerated by the largest global pandemic since the Spanish influenza in 1918, telehealth has been thrown into the spotlight during the COVID-19 pandemic. By integrating consumer-focused wearable health devices with smartphone apps and telehealth software platforms, a new age of disruptive innovation within the digital healthcare industry has arrived ahead of schedule. Immense potential lies in the digitization of arrhythmia diagnosis and treatment; however, its benefits are outweighed by valid concerns surrounding clinical validity, data security and regulatory policy. Our proposed framework for the integration of wearable devices into the clinician’s workflow can serve as a starting point for considerations on future use. As wearable technologies and biosensor capabilities continue to evolve, so too does the scope to manage cardiovascular pathology remotely in a safe, high-quality and cost-effective manner, allowing earlier investigation in patients presenting with their own data and, ultimately, decreasing the time to diagnosis and use of healthcare. As the first clinical trials begin to report cardiovascular outcome data rigorously, we can cautiously expect that consumer-provided wearable technology could become a valid tool in arrhythmia management, alongside traditional, yet evolved, precision medical-grade devices throughout the next decade, proving itself a paradigm shift rather than a pipe dream.







