Radiofrequency catheter ablation (RFA), introduced in the 1980s, represents a major advancement in the field of electrophysiology.1,2 With reported acute procedural success rates of up to 95 %, catheter-based ablation has become the standard of care for paediatric supraventricular tachycardias (SVTs).3,4 Traditionally, electrophysiology catheterisation studies including RFA have been guided fluoroscopically. However, exposure to ionising radiation potentially puts any patient at risk of deterministic effects, such as cataract, erythema and epilation, as well as stochastic effects, which include radiation-induced cancer and genetic alteration.5–7 This is particularly relevant in the paediatric population as the young are more radiosensitive. With a predicted longer lifespan, children are at a higher risk than adults for expressing the long-term stochastic effects of radiation.8 Non-fluoroscopic, three-dimensional (3D), electroanatomic mapping systems were initially used to guide electrophysiology studies in the late 1990s.9 3D imaging (3DI) initially served as an adjunct to fluoroscopy in catheter ablation among adult patients. Further refinements of the navigation systems have facilitated performance of RFA under the sole guidance of 3DI, thus eliminating radiation exposure entirely. SVT ablation using 3DI only has been reported in the paediatric population as well.10–12 To date, there are no studies of limited FI with 3DI usage that takes into account efficacy, procedure cost and anaesthesia duration. This study evaluates efficacy, time, cost and safety profiles of limited FI plus 3DI for SVT ablation in the young.
Methods
This was a retrospective study conducted at the Children’s Hospital of Michigan. The institutional review board and the Human Investigation Committee at Wayne State University School of Medicine, Children’s Hospital of Michigan/Detroit Medical Center approved the study. The electrophysiology database of our institution was reviewed to identify
all patients who underwent electrophysiology study (EPS)/ablation for standard forms of SVT between October 2009 and June 2012. To limit confounding variables, only patients with structurally normal hearts were included in the study. Also, ablation for only standard forms of SVT, including atrioventricular reciprocating tachycardia (AVRT), AV node re-entry tachycardia (AVNRT) and atrial ectopic tachycardia (AET), was compared. In this regard, patients with more complicated tachyarrhythmias, including atrial flutter and fibrillation and congenital heart anatomies, were excluded in order to maintain better consistency in evaluation. Also, those patients in whom simple forms of SVT, as above, degenerated into atrial flutter or fibrillation were excluded.
Demographic, clinical, electrophysiology and follow-up data were collected. This included age, gender, height, weight, type of SVT, presence of preexcitation on baseline electrocardiogram, location of any accessory pathways, fluoroscopy time, radiation dose area product (DAP) incurred to the patient, EPS procedure time, total general anaesthesia (GA) time, procedural cost, acute procedural success and any associated complications. Any recurrence of SVT or preexcitation or other complications at a follow-up interval of up to 30 months were also recorded.
Electrophysiology Procedure
A 15-lead electrocardiogram and an echocardiogram were obtained for all patients during pre-procedure evaluation. Informed consent for the electrophysiology procedure was obtained from the parent or patient as appropriate. Any antiarrhythmic medications were discontinued at least 5 half-lives prior to the procedure. For all patients, EPS or ablation was performed under GA as per institutional protocol. In all cases, the EnSite NavX™ (St. Jude Medical, St Paul, MN, US) electroanatomic system was used with NavX™ skin patches. Both of our two electrophysiologists combined a conscious awareness to use only limited fluoroscopy associated with 3DI (FI plus 3DI) to guide the procedure for mapping, ablation, venous concerns and transseptal procedures when required. After obtaining femoral venous access, electrophysiology catheters were placed in traditional atrial, coronary sinus and ventricular inflow regions. Fluoroscopic use was determined individually by each physician based on subjective need to view catheter position, venous anatomy or during transseptal procedures. In use of limited fluoroscopy at 15 cycles per second (cps) and lower, the quick step-on and step-off technique was used to minimise the radiation exposure. Since our institution has a Cardiology Fellowship Training program, all procedures entailed some degree of Fellow education involving FI and 3D mapping with catheter manipulation.
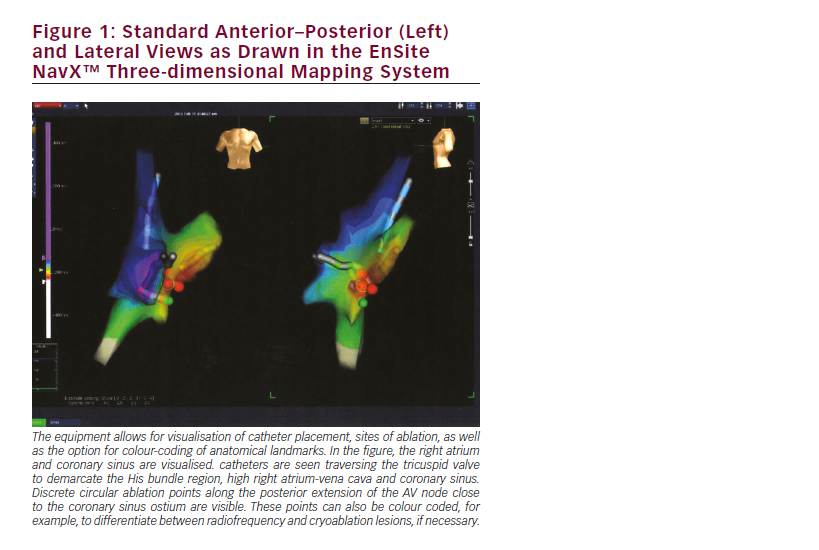
Conventional anteroposterior and lateral fluoroscopic images were obtained and the last-image-hold feature was utilised. Catheter placement was performed using the conventional technique that has been previously described.13 Using a standard, flexible, quadripolar catheter detailed geometries of superior vena cava, inferior vena cava, right atrium, coronary sinus and tricuspid valve annulus were then obtained. Following construction of cardiac chamber geometry, biplane views of the EnSite NavX™ system were used for navigation (see Figure 1). Activation, voltage and pace mapping were utilised as needed. The EPS was performed using established pacing protocols both with and without isoproterenol infusions. In the event of haemodynamic instability associated with induced tachyarrhythmias, the patient was cardioverted. After delineating the SVT mechanism and substrate, ablation was performed using radiofrequency and/or cryoablation energies per physician preference and anatomic location. The ablation sites were marked in the 3DI system, and additional application of energy was performed at and around the site of initial energy application. For patients with left-sided pathways, transseptal puncture was performed. After ablation, intravenous adenosine was administered to obtain transient AV block to evaluate for any residual accessory conduction. After a waiting period of 60 minutes post-ablation, repeat programmed electrical stimulations (PESs) were performed with and without isoproterenol infusions. Additional ablation was performed only if tachycardia was re-induced. The electrophysiology procedure was considered acutely successful if, after 60 minutes following the last ablation, there was no inducible tachycardia on PES, the QRS complex was normal without evidence of preexcitation and there was absence of prograde conduction on blocking the AV node with adenosine.
The total fluoroscopy time for the procedure took into consideration usage of anteroposterior and lateral cameras. The total radiation DAP was measured using the inbuilt DAP metre (Axiom Artis™, Siemens Healthcare, Malvern, PA, US). This was expressed in milligray-m2 (ìGy-m2). GA time was defined as the time from induction of anaesthesia to postprocedure extubation in the electrophysiology lab. The electrophysiology procedure time was defined as the time from completion of vascular access to the time of removal of the vascular sheaths. Per our institutional finance protocols, the finance charge for catheterisation laboratory usage and electrophysiology and ablation studies was the same irrespective of catheter guidance modality used or duration of study. However, the procedural charge for GA varied depending on the duration of the procedure. The GA cost was calculated using a base amount with additional charges per unit time that GA was utilised. Therefore, the procedural cost was defined as the total cost incurred for GA.
After the electrophysiology procedure, the patients were monitored overnight on telemetry per institutional policy. An echocardiogram was obtained the day after the procedure to evaluate for any pericardial effusions and atrioventricular valve function. A 15-lead electrocardiogram was performed to ascertain QRS morphology prior to discharge. The patients were followed at 1–2 weeks, 6 months and then yearly after the procedure to evaluate for recurrence of any arrhythmias. Follow-up data collection included history of recurrence of any arrhythmia-related symptoms, results of 15-lead electrocardiograms including persistence or recurrence of preexcitation even without symptoms, exercise stress testing, event monitor or repeat EPS results as appropriate.
Statistical Analysis
This was a retrospective study. All data were reported as mean and standard deviation (SD) for continuous variables and frequency for categorical variables. All statistical analyses were performed using Graphpad prism software (Graphpad Inc, CA, US). Statistical significance was set at a p value of less than 0.05.
Results
A total of 81 patients with structurally normal hearts underwent EPS for the mentioned standard forms of SVT at our institution between October 2009 and June 2012. Demographic and procedural data are shown in Table 1. Of this study population, 47 patients (58 %) were males and 34 (42 %) were females. The mean patient age at the time of the electrophysiology procedure was 13.2 ± 3.4 years. The mean body surface area was 1.56 ± 0.36. AVRT was seen in 60 patients (74 %), AVNRT in 12 (14 %) and AET in 4 patients (2.4 %). Both AVRT and AVNRT were detected in seven patients (8.6 %). Among patients with accessory pathways, 27 (41 %) were right sided and the remaining 40 (59 %) were left sided.
The acute post-procedural success rate, as defined above, for all patients was 93.8 %. Five patients exhibited residual mild preexcitation; however, tachycardia was not re-inducible in spite of extensive pacing plus isoproterenol infusion. All five of them exhibited extensive rightanterior and anteroseptal accessory pathways.
Total fluoroscopy time among all patients was 10.07 ± 6.8 minutes. Total patient radiation DAP was 2.47 ± 2.78 mGy-m2. The two electrophysiologists who performed the procedures differed by experience (>20 versus <10 years; p<0.01). Details of the electrophysiology procedures based per physician are shown in Table 2. Comparison of the electrophysiology procedure time, including the 60-minute post-ablation waiting period, showed that studies were significantly longer depending on physician experience (145 ± 36 versus 293 ± 78 minutes; p<0.001). As a result, total GA time was also significantly different between physicians with the less-experienced physician, as expected, requiring significantly longer duration of GA (367 ± 76 versus 211 ± 35 minutes; p<0.001). The electrophysiology catheterisation study charge was the same irrespective of the total study duration. Therefore, procedural cost for GA was higher depending on study duration ($3,348 ± 509 versus $2,309 ± 238; p<0.001). All studies included assistance by cardiology fellows-in training. Time spent on teaching catheter manipulation techniques was comparable between established electrophysiologists.
There were no procedure-related deaths. However, one complication occurred: complete heart block with junctional escape rhythm, associated with anteroseptal accessory pathways. During the mean follow-up of 18 months (range: 6–30 months), a total of 11 patients (14.4 %) had recurrence of tachycardia or preexcitation. Of the seven patients with tachycardia, four exhibited multiple anterolateral and anteroseptal accessory pathways, and three had left posterior accessory pathways. Two of the patients with recurrent tachycardia underwent a successful second procedure, while the condition of others was managed medically. Recurrence of preexcitation occurred in four patients.
Discussion
SVT is the most common form of tachyarrhythmia in children. Catheter ablation has substantially improved the outcome in these patients. Currently both fluoroscopy and non-fluoroscopic, 3D mapping systems are available to guide catheter ablation of SVT. 3D electroanatomic mapping systems have been shown to reduce the use of fluoroscopy during catheter ablations. The question remains as to whether nonfluoroscopic systems should be used exclusively to reduce any radiation exposure or used as a combination imaging system with minimal exposure. Both concepts have inherent risks and benefits. At present, this argument continues and decisions are based on user preferences. Although previously published reports have indicated that it is feasible to perform the entire electrophysiology mapping and ablation procedure using guidance by 3DI alone.10,11,14 In our institution, the electrophysiology catheterisation protocol allowed for individualisation of the extent of usage of 3DI based on the preferences of the electrophysiologist performing the procedure.
In our current study, the overall acute success rate among all 81 patients using limited FI plus 3DI was 93.5 %. This is comparable to previously published reports. The paediatric RFA registry reported an overall success rate of 90.4 % in the early era (1991–1995) and 95.2 % in the late era (1996–1999) for catheter ablation of paediatric SVT.4 For ablation of AVRT, our success rate was 92 %. Of the seven cases of AVRT that were not completely acutely successful, six had extensive right anteroseptal and one had multiple posteroseptal fibre locations. Previous literature has demonstrated lower success rates for such right-sided pathways in comparison with left-sided locations.4 All cases of AVNRT and AET that underwent ablation were acutely successful.
The paediatric radiofrequency ablation registry reported a mean fluoroscopy time of 40.1 minutes in the late era.4 With such long fluoroscopic exposure, there is potential for radiation-related skin injury. Moreover, this puts the patient at risk of long-term stochastic effects, such as radiation-induced cancer and genetic alterations. Advancements in fluoroscopic systems, use of pulsed fluoroscopy and increased operator awareness of the side effects of radiation have led to substantial reduction in the fluoroscopy time over the years. Currently available 3D mapping systems delineate anatomy more accurately and help to further reduce fluoroscopic exposure.15 The underlying principle of radiological protection is that the radiation dose must be as low as reasonably achievable (ALARA) in all instances of medical radiation exposure.16 Adhering to the ALARA principle, in studies where limited fluoroscopy was utilised, care was taken to keep the radiation dose as minimum as possible. Fluoroscopic guidance when used as a quick step-on and step-off technique limited fluoroscopy time in this study. It has been shown that the radiation dose can be substantially reduced by using ultra-low pulsed fluoroscopy rate of as low as 6.25 cps.17 In our study, the frame rate was kept at 15 cps or lower. Last-image-hold option was used. Our data show that fluoroscopy time can be kept to a minimum using 3DI along with limited fluoroscopy. This is comparable to results of previous studies. In a prospective study by Miyake et al., use of non-FI during paediatric SVT ablation resulted in substantial reduction of fluoroscopy time.18 Papagiannis et al. reported a significant decrease in fluoroscopy time with the use of EnSite NavX™ (10.2 ± 6.5 versus 27.1 ± 16.6 minutes) compared with conventional fluoroscopic guidance with no difference in the success or complication rates.14
Fluoroscopy time gives an estimate about the radiation exposure. However, for more accurate assessment of radiation risk, further quantification of exposure is necessary. DAP, which is the product of the dose in air in a given plane by the area of the irradiating beam, has been used for estimating the patient skin dose and the stochastic risk. Chida et al. reported that the radiation DAP correlates better with the actual dose that the patient receives than the fluoroscopy time.19 Casella et al. reported very low fluoroscopic exposure in adults undergoing ablation of SVT using a combination of 3DI and limited fluoroscopy. In the 24 % of cases in that study where the combination was used, radiation exposure was limited to 1.3 ± 1.1 mGy-m2.13 In a recent study, Gellis et al. showed that using a low fluoroscopy protocol based on the concept of ALARA, radiation dosage can be kept as low as 343.4 μGy-m2 during catheter ablations of paediatric SVTs.20 The acute success rate was 95 %, comparable to our study. However, the follow-up period was only 3 months and hence arrhythmia recurrence could potentially be underestimated. A recent study by Bhat et al. showed that even though the acute success rate of paediatric SVT ablation was high (97.5 %) among the 202 study subjects, the chronic success (5 years post-ablation) was only 75 %.21
In our study, the DAP for the limited FI plus 3DI procedures was 2.47 ± 2.78 mGy-m2 with an acute success rate of 93.4 % and recurrence rate of 14.4 % at a mean follow-up period of 18 months post-procedure. The dose of 2.47 mGy-m2 is comparable to the naturally occurring annual background radiation in US, which is 3.1 mGy/yr.22 Effects of such lowdose radiation are quite controversial. The linear no-threshold model states that any amount of radiation is harmful and has the potential to cause stochastic effects, such as induction of cancer.23 However, the conclusions are derived from extrapolation of data obtained from atomic bomb survivors during the Second World War who were exposed to large amounts of radiation in a short period of time.24 This is quite different from the medical radiation exposure scenario where the dose is quite small and the total dose is received over a longer duration of time. Computed tomography (CT) scans, which deliver very large cumulative doses of radiation, may be associated with some forms of cancer. A UK National Health Service study estimated that one excess case each of leukaemia and brain tumour per 10,000 head CT scans would occur.25 In general, however, definitive evidence for the damaging effects of doses in the range of 50–100 mSv is still lacking.26 To date, in spite of 3 decades of fluoroscopic-guided catheter ablation therapies for arrhythmias, there have been no definitive studies relating any increase in childhood cancers.
In our study, procedure duration and associated GA time and cost was a factor of physician experience. As can be expected with all interventional procedures, experience comes with time. It can therefore be anticipated that this variable would not be a factor in the future. Also, in any teaching institution, the need to educate future cardiologists in intravascular catheter manipulation adds time to any catheterisation procedure. Increased procedure duration time was reported in a comparison report by Miyake et al.,18 among studies using 3DI alone. In that study, the electrophysiology procedure time was significantly longer in the 3DI-only group, attributed to laboratory and equipment set up time as well as increased time for mapping. These were not factors in this study. In our institution, our electrophysiology laboratory is set up for 3D mapping in all cases and catheterisation laboratory procedure time is a single cost rate, irrespective of study duration. So, in this study, any time difference was related to physician experience.
Having immediate accessibility to FI inherently is associated with some advantages during any intracardiac electrophysiology or invasive catheterisation procedure. The need to ascertain venous patency, exact catheter position or, in rare instances, suspected cardiac perforation, for example, during transseptal punctures implies that use of limited FI does carry a definite benefit to patient safety.
As health care costs continue to rise, hospital systems are now trying to implement policies that would help decrease cost without compromising care. In our study, a conscious application of limited fluoroscopy combined with 3D cardiac imaging was associated with excellent results comparable to other reports and less radiation than reported previously for SVT ablation in the young. Procedure duration and associated cost was a factor of physician experience and is anticipated to decrease with time. However, since radiation exposure can be additive especially among patients who might require other diagnostic or therapeutic modalities, all factors need to be taken into account when informing patients of procedure risks and benefits.
Limitations
This was a single-centre retrospective study, involving two physicians, with a relatively small number of patients. Operator-dependent variables make generalisation of the results difficult. Further multicentre prospective studies will be needed to validate the findings.
Conclusion
As reported in this study as in others, catheter ablation with 3DI guidance can be safe and effective. However, improvements in FI with a conscious awareness to limit usage decreases radiation exposure time from previously reported studies, in addition to adding a potential patient safety valve, especially among institutions charged with the task of training future cardiologists. In the current era, a combination of both technologies may be seen as a more optimal way of ensuring ablation success while limiting potential complications. To decide which imaging technology or combined technologies to use, the potential adverse effects of limited radiation, need for additional procedures and additive effects of other future diagnostic and therapeutic modalities versus longer GA, procedure time and higher cost need to be weighed in any discussions with the patient’s family







