Introduction: The number of cardiac implantable devices continues to increase. With an increasing volume of implants and redo/upgrade procedures, monitoring procedural quality and outcomes remains important. Following the merger and establishment of Barts Heart Centre (BHC) in 2015, we have bi-annually audited device related complications and changed clinical practice accordingly. We report on our most recent annual device complication rates, which involved a more methodical auditing process.
Methods: All procedural workflow data was entered into a dedicated cardiac device database (MediconnectTM). A procedure report was always attached to each procedure and uploaded to a central network. Complications were tagged electronically to each procedure enabling a direct tracker to the original procedural implant and physician.
Each month all procedures and complications were extracted from the database, including revision procedures. These were compared with the catheter lab procedural list to avoid erroneous duplication. Each complication was interrogated to ensure accurate coding prior to analysis. Complications were described according to NICOR reporting guidance with acute (same admission) and late (discharge–15 months) complications.
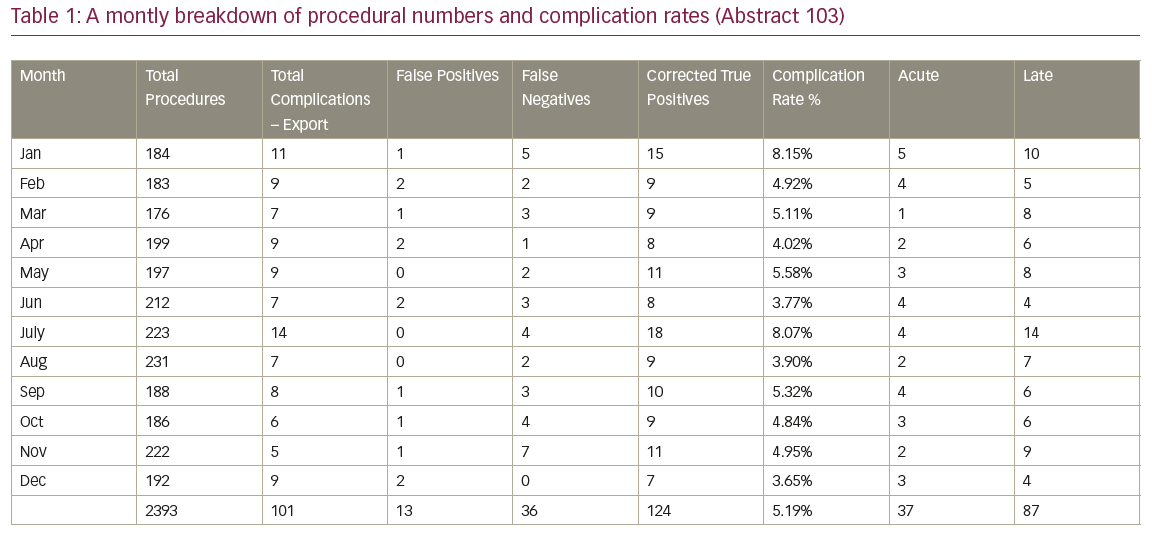
Results: Table 1 summarises the key data findings; 2,393 procedures were performed in 2018. The exported number of complications was 101, however there were 13 false positives and 36 false negatives bringing a total of 124 complications (absolute complication rate of 5.19%). Of these, 37 (29.8%) were acute and 87 (70.2%) were late complications.
The breakdown of procedure by device type were 451 Internal Loop Recorder (ILR), 1070 Permanent Pacemaker (PPM), 393 Internal Cardioverter Defibrillator (ICD), 85 Subcutaneous ICD (SICD) and 387 Cardiac Resynchronisation Therapy Device (CRT) procedures.
The top 10 most frequent were RV lead displacements: 32, RA lead displacements: 22, infection: 18, device discomfort/superficial re-site: 9, hematoma: 8, pneumothorax: 5, pericardial effusion: 4, device erosion: 4,
LV lead displacement: 3 and cardiac tamponade: 3.
A yearly comparison of complications showed a significant reduction; 2014 reported a 16% complication rate compared to 5.19% in 2018. This significant discrepancy may be explained by factors such as 2014 being
pre-merger of BHC, the introduction of MediconnectTM and a significant reduction of false positives as a result of improved awareness for data collection.
Limitations include under-reporting of complications not requiring procedural correction (e.g. hematoma requiring observation). Additionally, our database system only permits one complication per case therefore
cases with multiple complications required manual adjustments. Our clinical governance process has enabled improved accuracy and operator specific complication reporting. Retrospective accountability remains a culprit for under-reporting complications; patients inherently receive care from multiple disciplines, making a robust data tracker for auditing complications a difficult task.
Conclusion: We report the complication data of a large volume centre for cardiac device procedures. A methodical audit has helped identify false positives and negatives to improve quality assurance. Regular reporting of departmental complications and reflection on protocols has influenced departmental changes in practice.