Introduction: Implantable cardiac monitor (ICM) is a small device implanted to detect and record arrhythmias and symptomatic events. The procedure has a low complication rate, with procedures being performed in an outpatient setting. Due to greater accessibility to ICMs there has been an increasing amount of ICM devices on the market with new innovations in hardware and software. We sought to conduct a quality assessment of each device to evaluate each ICM with regard to procedural complications and remote monitor (RM) connectivity to provide a guide for NHS services.
Methods: Patients referred for ICMs for any indication were prospectively enrolled in a tertiary, adult cardiac centre. We aimed to implant 30 of each ICM: Medtronic (Reveal Linq, L1 & Linq II, L2), Abbott (Confirm Rx, CRx) and Biotronik (Biomonitor III, BMIII). Implants for each device were performed in series; 1st BMIII, 2nd L1, 3rd CRx, 4th L2, in an outpatient clinic area by experienced (50-250 procedures each) Specialist Nurses or Cardiac Scientists. Closure technique using wound closure strips, which were removed 7–10 days post procedure. Outcomes were assessed using electronic records, remote monitoring data and patient wound images. Remote monitoring was assessed at the 1-month check with patients being seen face to face (pre COVID-19) and virtually (post March 2020). The study was registered with the hospital clinical effectiveness unit.
Results: 94 patients were enrolled between January 2020 and April 2021: 32 CRx, 25 L1, 25 L2 and 12 BMIII. Outcomes can be seen in Table 1. Biomonitors implants were reduced due to a high protrusion rate (33%), resulting in them being withdrawn from QA assessment. Procedural complications showed 0 infections, with the only complication being device protrusion.
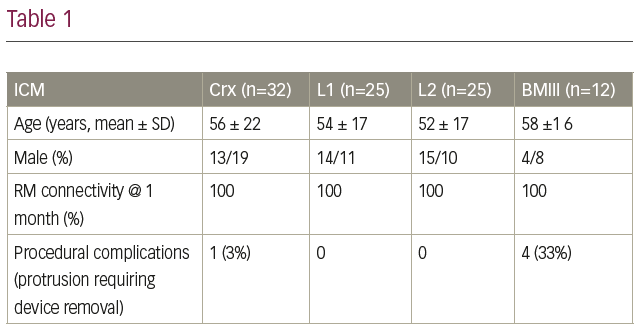
Discussion: Our data shows that there is a disparity in the type of device with the model of care we provide. The BMII has significantly more wound complications that other devices when the wound is closed with wound closure strips (WCS) (e.g. steri-strips). This is likely to be because the incision blade is wider than that of its counterparts, relying more on the WCS to support the wound. Compounding this, the device antenna design acts as a spring if any counter pressure is applied to it, such as a female patient wearing a bra, effectively pushing the device back towards the scar. There was also 1 complication in the CRx arm due to protrusion, which could be attributed due to its wider blade compared to L1/L2. However, 1 complication does not prove any systemic issues and should be assessed in a comparison with larger implant numbers. Remote monitoring connectivity has shown to be excellent across all manufacturers. There are external factors at play to produce these high results. Specifically, the L1 patients were enrolled on FocusOn with troubleshooting for connectivity utilising the BeConnected (Medtronic telephone support) service. Therefore, this does not attribute just the technology but also the service. Unfortunately, the L1 device does not download full disclosure of recordings in contrast to the L2, CRx and BMIII, which send all recordings and were connected prior to departure. RM should be assessed on a wider cohort from multiple centres over longer periods.
Conclusion: Our findings show there is a good compliance with RM connectivity at 1 month with all devices. The BMII having significant wound complications if used with WCS. We would recommend BMIII is utilised with stitches to secure the deep layers of the wound.







