Introduction: Adverse drug reactions (ADRs) are responsible for 6% of annual hospitalizations and 2% of deaths, representing a significant burden on the healthcare system. Cardiovascular drugs are responsible for most ADRs, among which bradyarrhythmias are frequent outcomes. Recent studies suggest that in most cases of drug-associated atrioventricular (AV) conduction disorders, a subclinical dysfunction of the conductive tissue may be involved. Identifying risk factors and developing a predictive model for bradyarrhythmias associated with bradycardic drugs would represent a valuable tool for clinical practice.
Material and methods: We conducted a retrospective cohort study on 686 patients with a primary diagnosis of symptomatic bradyarrhythmia admitted to a single tertiary referral center. The patients were divided into two groups based on bradycardic treatment: under medication (n=343, divided as follows: beta-blockers 55% of cases, digoxin 21%, amiodarone 15%, propafenone, calcium channel blockers, sotalol and ivabradine – less than 10% of cases), and without any type of bradycardic medication (n=343). We analyzed demographics, clinical and paraclinical parameters related to the identified AV conduction disorder. A multivariate regression analysis was performed to explore factors associated with medication use. Statistical analysis was performed using STATA 16 SE and SPSS statistics software.
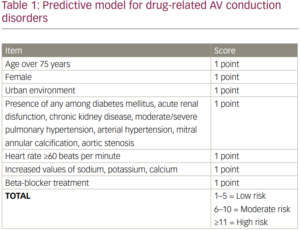
Results: The average age was approximately equal in the two groups (73.69 ± 8.82 vs. 74.0 ± 8.97), with a predominance of female patients (p=0.001). Patients on bradycardiac medication were associated with slow atrial fibrillation (p=0.001), sick sinus syndrome (p=0.917), sinus bradycardia (p=0.009), and sinus pauses (p=0.001), while third-degree AV block was more commonly found in the second group (p=0.001). Patients in the first group had higher mean systolic blood pressure values (p=0.007), but lower mean heart rate values (p=0.001). Medication use was associated with renal dysfunction (p=0.001), syncope (p=0.011), heart failure (p=0.010), severe left ventricular dysfunction (p=0.001), pulmonary hypertension (p<0.001), atrial dilatation (p=0.001), and the need for temporary cardiac pacing (p=0.022). Bradycardic drugs were associated with higher serum levels of potassium, (p=0.017), creatinine (p=0.044), and glucose (p=0.014), while patients in the second group had higher serum levels of sodium (p=0.001). Based on the multivariate regression analysis results, we developed a predictive model (Table 1) for the association of medication and AV conduction disorders (1 point each parameter, risk categories: low, moderate and high – AUC value 0.631, p=0.001).
Conclusions: AV conduction disorders associated with bradycardic drugs are a public health issue. Validation of a clinical model to predict which patients are at risk of developing such disorders can improve patient management.